Archives of Environmental Health, January/February
1981 (Vol. 36, No.1)
Changes in the Mineral Composition of Food as a Result of
Cooking in “Hard” and “Soft” Waters
B. S. A. HARING
W. VAN DELFT, B.Sc.
National Institute for Water Supply
The Netherlands
ABSTRACT. During the last decade many
epidemiologists have found an inverse relationship between water
hardness and cardiovascular mortality. It seems unlikely that
this relationship can only be attributed to a deficiency of
calcium and magnesium in drinking water, because only 10-20% of
the total daily intake of calcium and magnesium is derived from
drinking water. The purpose of this study was to investigate
changes in the mineral composition of food when cooking with
waters of different hardness. The most significant differences
were found for calcium; the concentration of this element in
potatoes and vegetables usually increased when cooking with
hard-water types, while a decrease was noted when soft water was
used for cooking.
THE TERM “hardness of water” is derived from the
characteristics of cations such as calcium (Ca), magnesium (Mg),
iron (Fe), manganese (Mn), aluminum (Al), and strontium (Sr) to
form insoluble compounds with soap. Calcium and Mg are the major
constituents in drinking water that contribute to its hardness.
Hardness is usually expressed in terms of the equivalent
concentration of calcium carbonate (CaCO3). Drinking water hardness exceeding 150 mg/L
(ppm) as CaCO3 is considered to be
“hard.” Statistical evidence indicates that death
rates from cardiovascular diseases are inversely correlated with
the hardness of drinking water.1-4
Three possible explanations for the observed statistical
associations follow.
1) Calcium and/or Mg intake by consumption of hard drinking
water helps meet the total dietary requirements for these
essential elements, and may in turn, suppress the toxic effect of
some heavy metals.1,2
2) Heavy metals in soft drinking water [e.g., cadmium (Cd) and
lead (Pb)] released from piping, resulting in supposedly higher
corrosiveness of the softer waters during distribution, have
toxic effects.5-9
3) While cooking food, “soft” water extracts more
essential elements (e.g., Ca and Mg) from vegetables and potatoes
than “hard” water.10
The second above listed explanation seems unlikely,
particularly in The Netherlands, because significant positive
correlations were found between the hardness of drinking water
and the concentration of metals—Cu and Pb—released
from lead and copper water distribution pipes.14
The purpose of this study is to investigate changes in the
mineral content of foods when boiled in waters of different
hardness. This type of study was recommended by a World Health
Organization (WHO) working group on health effects of the removal
of substances naturally occurring in drinking water.11 The total daily intake of Ca and Mg from an
average diet is mainly derived from milk, bread, and vegetables.
The estimated intake of Ca and Mg from 2 L drinking water per day
is usually less than 10-20% of the total daily intake.1 Although Ca and Mg are readily absorbed from
water, the intake of Ca and Mg from food, considering the effect
of cooking in waters of different hardness, may contribute a
plausible explanation for the observed inverse statistical
association between cardiovascular disease mortality and hardness
of drinking water.10
MATERIALS AND METHODS
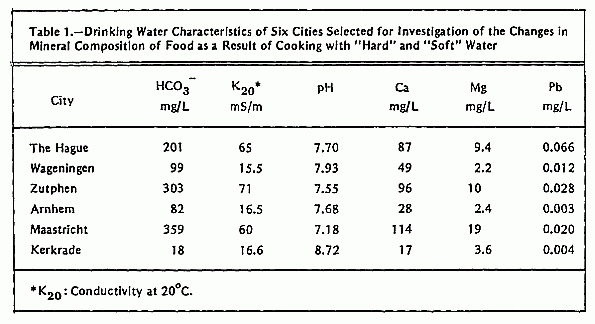
Water for the cooking experiments was sampled at the tap at
five different localities in each of six selected water supply
areas: 1) The Hague, 2) Wageningen, 3) Arnhem, 4) Zutphen, 5)
Maastricht, and 6) Kerkrade. In this way a representative sample
of drinking water for each city was obtained. Data on hardness,
conductivity, HCO3- and pH of these
water samples are given in Table 1. For the cooking experiments
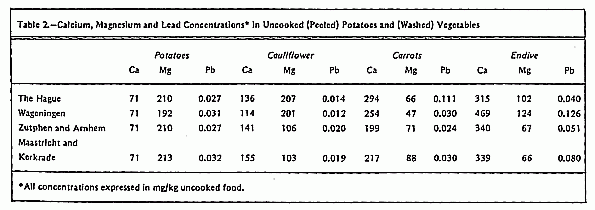
the following foods were chosen: potatoes, cauliflower, carrots,
and endive. These foods can contribute considerably to the
average daily intake of Ca and Mg12, 13
(Table 2).
All cooking experiments were conducted in duplicate.
One-hundred-fifty grams of all examined foodstuffs were cooked in
beakers with 250 ml water and 1.25 g NaCl. After draining, the
cooked potatoes and vegetables were freeze-dried and ground.
Weighed portions of the freeze-dry samples were ashed with
sulfuric acid (H2SO4) at 500°C; the resulting white ash was
dissolved in hydrochloric acid (HCl).
The water that remained after cooking was adjusted to the
original 250 ml volume with double distilled water to correct for
losses by evaporation. After centrifugation, the solution was
refluxed with nitric acid (HNO3) and
hydrogen perioxide (H2O2) to destruct organic compounds which might
interfere with the elemental analysis by atomic absorption
spectroscopy. A Perkin Elmer model 373 flame atomic absorption
spectrometer was used for determination of Fe, Mn, Ca, Mg, sodium
(Na), potassium (K), and zinc (Zn) in the destructed samples of
food and water. Trace elements, i.e., Cd, Pb, were determined by
anodic stripping voltammetry using a PAR model 374 polarographic
analyzer.
RESULTS AND DISCUSSION
The concentrations of Ca, Mg, Fe, Na, K, Zn, Cd, and Pb were
determined in both food and water samples before and after
cooking. A selection of data on Ca, Mg, and Pb is presented in
Figure 1 and Table 2. The calcium concentration in potatoes and
vegetables usually increased when cooking with hard water types,
while in most cases it decreased when soft water was used for
cooking. The results of the Mg analyses in food and water show
that in all cases Mg is extracted from the food. This effect was
slightly pronounced when the food was prepared with soft water
types. The Pb concentrations in food were found to be higher
after cooking with hard water and lower when soft water was used
for cooking. These differences, however, were probably caused by
the higher Pb concentrations in the hard water types (Table 1).
The concentration in the examined foodstuffs of elements such as
Fe, Mn, K, and Zn were all lower after cooking, regardless of the
water hardness. A few cooking experiments were repeated under the
same conditions as described above except for the addition of
table salt. The results of these tests were approximately the
same as for the other experiments.
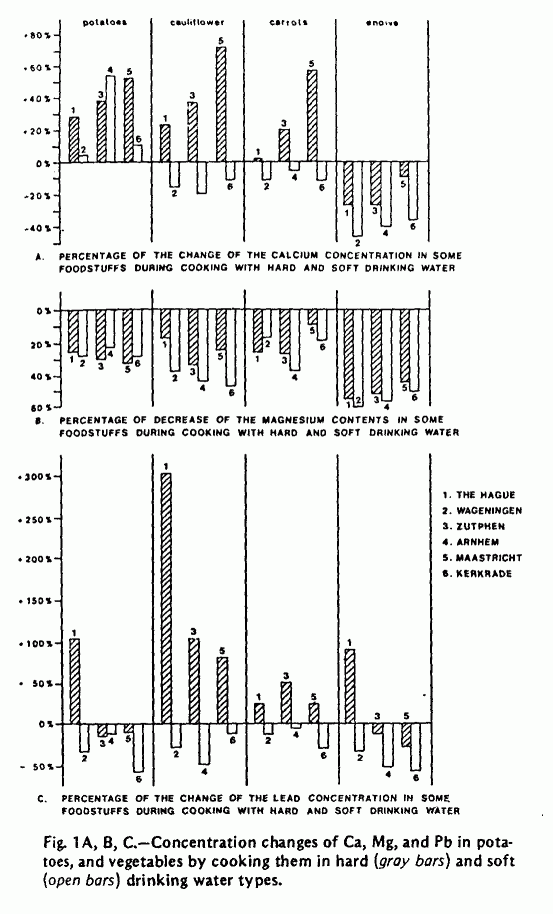
The results of the cooking tests, with special reference to
the changes in Ca and Mg concentration when food is cooked in
hard and soft water, support the theory that the statistical
inverse relation found between hardness of drinking water and
cardiovascular disease mortality is possibly caused by a
deficiency of these elements in soft drinking water. Such a
deficiency will be further increased by the extraction of Ca and
Mg from food by soft waters. A more extensive study of the
factors affecting changes in the mineral composition of food
during cooking is recommended before final conclusions can be
drawn.
This study was conducted under contract no. 273-77-1 ENV-N of
the European Communities Environmental Research Programme.
Submitted for publication July 10,1980; accepted for
publication August 11, 1980.
Requests for reprints should be sent to: Dr. B. J. A. Haring,
National Institute for Water Supply, P. 0. Box 150, 2260 A.D.
Leidschendan, The Netherlands.
REFERENCES
1. Neri, L. C., and Johansen, H. L. 1978. Water hardness and
cardiovascular mortality. Ann N V Acad Sci 304:
203-19.
2. Amavis R.; Hunter, W. J.; and Smeets, J.G.P.M. 1975.
Hardness of Drinking Water and Public Health. Proceedings of
the European Scientific Colloquium, Luxembourg, May 1975.
Oxford: Pergamon Press.
3. Crawford, M. D. 1972. Hardness of drinking water and
cardiovascular disease. Proc Nutr Soc 31: 347-52.
4. Biersteker, K., and Zielhuis, R. L. 1975. Hard of zacht
drinkwater. Tijdschr Soc Geneesk 53: 3-9.
5. Schroeder, H. A., and Kraemer, L. A. 1974. Cardiovascular
mortality, municipal water and corrosion. Arch Environ
Health 28: 303-11.
6. Elwood, P. C.; St. Leger, A. S.; and Morton, M. 1976.
Dependence of blood lead on domestic water lead. Lancet
1: 1295.
7. Beattie, A. D.; Campbell, B. C.; Moore, M. R.; and
Goldberg, A. 1976. Blood-lead and domestic water lead.
Lancet 2: 200-01.
8. Pocock, S. J. 1980. Factors influencing household water
lead. Arch Environ Health 35: 45-51.
9. Anonymous. 1978. Progress in the water story. Br Med
J 1:264.
10. Dauncey, M. J.; and Widdowson, E. M. 1972. Urinary
excretion of calcium, magnesium, sodium and potassium in hard and
soft water areas. Lancet 1: 711-14.
11. World Health Organization. 1979. Health effects of the
removal of substances occurring naturally in drinking water, with
special reference to demineralized and desalinated water. Report
on a Working Group, Brussels, 20-23 March 1978. Euro Reports and
Studies 16. Copenhagen: World Health Organization.
12. United States Department of Agriculture. 1975.
Handbook of the Nutritional Contents of Foods. New York:
Dover.
13. Nederlandse Voedingsmiddelen Tabel. 1979.
‘s-Gravenhage: Voorlichtingsbureau voor de Voeding.
14. Haring, B.J.A., and Zoeteman, B.C.J. 1980. Corrosiveness
of drinking water and cardiovascular disease mortality. Bull
Environ Contam Toxicol 25: 658-62.
This page was first uploaded to The Magnesium Web Site on
September 20, 2002
http://www.mgwater.com/



