Reprinted from THE JOURNAL OF REPRODUCTIVE MEDICINE, Vol. 32
No. 6, June 1987
Role of Nutrition in Managing the Premenstrual Tension
Syndromes
Guy E. Abraham, M.D.
Ruth E. Rumley, M.D.
The clinical, biochemical and endocrine effects of a total
dietary program were evaluated in patients with the premenstrual
tension syndromes (PMTS). The program consisted of dietary
guidelines and nutritional supplementation. Open trials suggested
that an initial dosage of the supplement consisting of six
tablets daily gave the best symptomatic relief during the first
three to six months. Double-blind studies confirmed that a daily
average of six tablets decreased PMTS symptom scores to
significantly lower levels than did the placebo. A significantly
higher percentage of PMTS patients reported feeling better on the
dietary program than did those on the placebo. Although
significant changes were observed in some liver function tests,
the values were within the normal ranges. The dietary program,
implemented for three to six months, decreased serum estradiol
17-β and increased serum progesterone levels during the
midluteal phase in PMTS patients. Nonresponders using the program
should be reevaluated and treated according to the results of the
reevaluation and the PMTS symptoms.
Introduction
In a recent survey of 502 U.S. physicians,81 90%
said that they provided and 60% said that they recommended diet
and nutritional supplements in the management of the premenstrual
tension syndromes (PMTS). However, the kind of dietary
recommendations made by those physicians and the rationale for
the recommendations were not given. There is no general agreement
on the nutritional program best suited to PMTS patients. We have
proposed dietary recommendations based on PMTS symptom
subgroups.5-7 Our purpose here is to define the PMTS
subgroups and our nutritional program and to evaluate the
clinical, biochemical and endocrine effects of this regimen on
PMTS patients.
PMTS Subsyndromes
Although 150 symptoms have been added to the list of PMTS
symptoms since Frank’s original publication88,
120 on the subject, most are not common and usually
represent an exacerbation of a preexisting condition. The most
common symptoms for which PMTS patients seek medical advice and
relief can be divided into four subgroups.6 Each
subgroup may exist alone or in combination with other
subgroups.
PMT-A
The chief complaints of patients in the PMT-A category are
anxiety, irritability and nervous tension, occurring as early as
the midcycle, becoming progressively worse during the luteal
phase, sometimes followed by mild to moderate depression and
improving with menses. This PMTS subgroup is the most common one.
Stieglitz and Kimble 116 found it in 68% of PMTS
patients evaluated; Morton,90, 91 in 100%;
Rees110 in 73-80%; and Mukherjee,93 in 77%.
We found a prevalence of 66% in 702 PMTS patients
evaluated.61
PMT-H
The PMT-H subgroup is characterized by a premenstrual
sensation of weight gain, abdominal bloating and tenderness,
breast congestion and mastalgia, and occasionally edema of the
face and extremities. The actual premenstrual weight gain is
<3 lb except in severe PMT-H, in which a premenstrual weight
gain >5 lb is observed. With increased age, the weight gain is
not completely lost with menses, and problems with overweight
occur.
This subgroup is the second-most-common PMTS subgroup and
occurs in 60-66% of PMTS patients. Mukherjee found a 60-66%
incidence of PMT-H in his PMTS patients. Sutherland and
Stewart118 observed a PMT-H prevalence of 63%. We
found PMT-H in 65% of PMTS patients.61
PMT-C
The PMT-C subgroup is characterized by an increased
premenstrual appetite and craving for sweets (mainly chocolate),
with subsequent ingestion of large amounts of refined sugar,
followed a few hours later by fainting spells, fatigue,
palpitation and headache. This indulgence in eating sweets occurs
during stressful situations, or at least in situations perceived
by the patient as stressful. Smith and Saunder115
found a positive correlation between a premenstrual craving for
sweets and a feeling of tension. Liebowitz and Klein79
postulated that the craving for chocolate is due to its high
content of phenylethylamine and that these patients are deficient
in central nervous system phenylethylamine, a psychotropic
substance believed to act by stimulating dopamine release. Morton
observed PMT-C symptomatology in 44% of PMT sufferers. We have
found a prevalence of 24% in women suffering from PMTS
symptomatology
PMT-D
In PMT-D patients, the PMTS is characterized by premenstrual
depression, withdrawal and suicidal ideation followed by a
suicide attempt. PMT-D patients complain of being lethargic,
confused and incoherent and of having difficulty verbalizing.
They usually do not seek medical care on their own but are
brought to a psychiatrist by concerned friends or relatives. This
PMTS subgroup, in the absence of PMT-A, requires psychiatric
consultation. Tonks120 observed that suicide attempts
and suicides occur premenstrually mote often in PMT-D patients
who do not complain of experiencing PMT-A symptomatology than in
PMT-D patients who do.
Fortunately, PMT-D frequently follows PMT-A and rarely occurs
alone. Stieglitz116 reported depression to occur in
36% of 67 PMTS patients but stated that the depression was
associated with irritability. Mukherjee93 found
depression in 13.5% of 74 patients evaluated, but PMT-D was the
prominent symptomatology in only 1.3% of the patients. We found a
1.7% prevalence of pure PMT-D and a 23% prevalence of combined
PMT-A and PMT-D.61
Diagnosis
A menstrual symptom questionnaire (MSQ) and a menstrual
symptom diary (MSD) were developed for screening and evaluating
PMTS.6 The criteria for PMTS classification require
that there be a symptom-free interval for at least one subgroup
during the week following the end of menses and a worsening
during the luteal phase, with symptoms rated premenstrually as
moderate to severe.
The postulated pathophysiology of the PMTS sub groups has been
described.5-8
Screening for Serious Medical Problems in PMTS Patients
Prior to initiation of the nutritional program, the
possibility of serious medical problems and life threatening
diseases should be ruled out with a careful history, complete
physical examination and routine hematology, urinalysis and blood
chemistry.6 Psychologic testing during the follicular
phase of the menstrual cycle will assist in detecting any
underlying psychiatric problems. A nutritional survey is
warranted in every PMTS patient prior to treatment since any
dietary recommendation must take into account prior dietary
habits and life-style. Because the intake of calories, fats,
carbohydrates and proteins fluctuates during the menstrual cycle,
with significant increases premenstrually, 9, 43 the
nutritional survey should be performed during both phases of the
menstrual cycle. There is also a significant increase in energy
expenditure premenstrually127that could explain
discrepancies in the reported caloric requirements of healthy
women.98
PMTS patients should be checked carefully for alcoholism. It
has been estimated that there are 2 million alcoholic women in
the reproductive-age group in the United States.42
Sixty-seven percent of alcoholic women relate their drinking to
the menstrual cycle, and drinking bouts occur almost always
during the premenstruum, supposedly to relieve anxiety and
nervous tension.22
A careful breast examination is warranted, not only to detect
mammary dysplasia but also to screen for breast cancer. Patients
with PMTS, mainly those scoring high for PMT-A, have a luteal
phase deficiency.10, 19, 32, 94 One prospective study
of 1,083 infertile patients revealed that the risk of breast
cancer was 5.4 times greater in women with a luteal phase
(progesterone [P]) deficiency than in those with a normal luteal
phase and normal P levels.36 Therefore, PMT A patients
should be considered at high risk for breast cancer. The
prognosis seems to be better when breast cancer is detected
during a routine examination by a physician than when discovered
accidentally.30
Determination of serum prolactin and thyrotropin releasing
hormone and a thyroid hormone evaluation are indicated in PMTS
patients with galactorrhea to rule out pituitary and thyroid
abnormalities.62, 71 Serum P and estradiol-17β
(E2 during the midluteal phase may assist in assessing
an adequate luteal phase in PMTS patients with unexplained
infertility and may serve as a guideline before and during
different treatment modalities.14 The role of serum
and red blood cell magnesium is still at the research stage, and
more clinical studies are needed to assess the mineral’s
usefulness in the evaluation and treatment of
PMTS.2-12
In PMTS patients with hirsutism, an adrenal suppression test
is indicated to rule out life-threatening pathology of the
adrenal cortex and ovary, to locate the source of the androgen
excess and to guide therapy.13 Some PMT-D patients
with hirsutism respond very well to the suppression of adrenal
androgens. Increased peripheral E2 and a concomitant
clinical improvement in depression are observed in such
cases.7
Hyperandrogenized PMTS patients do not respond well to the
nutritional program unless it is combined with androgen
suppression of the ovary and/or the adrenal
cortex.3
Rationale for the Nutritional Program
The nutritional program we recommend is based on dietary
surveys of normal women and PMTS patients,55 the
postulated pathophysiology of PMTS6 and the most
recently published data on the role of nutrients and their end
products on PMTS-related symptomatology.3-6
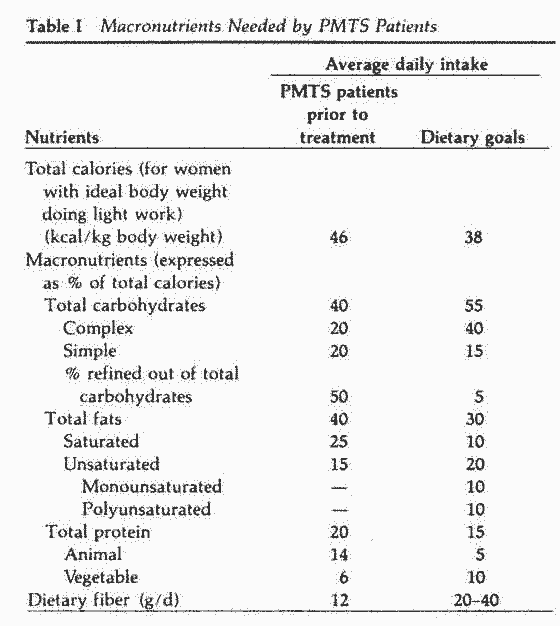
Macronutrients
A nutritional survey of 14 normal women and 39 PMTS patients
revealed a significant difference in the consumption of some
macronutrients.55 The PMTS patients consumed more
refined sugar, refined carbohydrates and dairy products than did
the normal women. Women with PMT-A symptoms consumed more
protein, more dairy products and more refined sugar than did PMTS
patients without PMT-A.2 Although the percentage of
calories from fats and proteins was not significantly different
between PMTS patients and normal women, the sources of fats and
proteins were predominantly vegetable products in normal women
and animal in PMTS patients. Dietary fiber intake was twice as
high in normal women, with an average of 12 g/d in PMTS patients
(Table I).
The dietary recommendations for macronutrients displayed in
Table I can be translated into some acceptable menus with the
help of a dietician or nutritionist. Food preferences and
allergies should be taken into consideration when preparing such
menus.
Carbohydrates. Carbohydrates can be divided into
complex (starch, glycogen, fiber) and simple sugars
(monosaccharides and disaccharides).25 Complex
carbohydrates can be divided into digestible (calorigenic) and
nondigestible carbohydrates (fiber).
Dietary fiber plays an important role in the digestion and
absorption of macronutrients and micro- nutrients, the metabolism
of toxins and steroid hormones, and colonic functioning.38,
47, 56, 72, 111, 124 Although an adequate fiber intake is
important for good health, an excess intake may be detrimental to
the absorption of certain trace elements.95 A daily
intake of 20-40 g of dietary fiber is recommended for PMTS
patients.
Patients with PMT-A symptomatology have elevated serum
estrogen levels.5-25 Food fiber increases the
intestinal clearance of estrogen. A recent study analyzed
peripheral and fecal estrogen and fiber in take in ten vegetarian
and ten omnivorous women who were menstruating
regularly.56 The omnivorous women consumed 11-13
g of fiber per day as compared to 25-33 g per day for vegetarian
women. A significant negative correlation between fiber intake
and blood estrogens and a positive correlation between fiber
intake and fecal estrogen suggest that food fiber increases the
clearance and fecal excretion of estrogen. Blood estrogen levels
were significantly lower in the vegetarian women than in the
omnivorous ones. Since women with breast cancer consumed
significantly less fiber than vegetarian
controls,15-64 and since women with severe
constipation from a lower fiber diet have a high prevalence of
precancerous lesions of the breast,101 this finding
has physiologic and clinical significance.
We recommend that 15% or less of the total calories be derived
from simple carbohydrates and that complex vegetable
carbohydrates represent at least 40% of the calories
consumed.
Simple carbohydrates ingested in large quantities have
deleterious effects on all PMTS subgroups. In PMT-C patients,
complex carbohydrates are preferred over simple sugars because
they stimulate insulin release in a less abrupt and more
sustained manner. A comparison of solid and liquid forms of sugar
revealed that sugar ingested in solution has a greater effect on
insulin release than when ingested in solid
form.114
An increased consumption of simple carbohydrates favors the
transfer of tryptophan from plasma to the central nervous
system.132 It will result in a central nervous
system (CNS) serotonin dominance, mainly if plasma free
tryptophan is elevated from a meat-containing diet. The increase
in CNS serotonin results in nervous tension, drowsiness, an
inability to concentrate and water retention.6
Ketoacid formation occurs when insulin is low and the
ingestion of simple carbohydrates minimal.7
After the ingestion of large amounts of sugar, the insulin level
increases abruptly, suppressing ketoacid formation and thereby
causing sodium and water retention, extracellular fluid volume
expansion and PMT-H symptoms. Sodium retention following the
ingestion of sugar is resistant to aldosterone inhibitors and
shows little correlation with the aldosterone secretion
rate.50 Insulin-induced sodium retention plays a
significant role in some PMT-H patients. In such cases, PMT-H may
be present in spite of normal and low aldosterone levels. By one
to two days, weight gain follows the overconsumption of sweets.
Besides the ketoacid mechanism of sodium retention by
sugar-triggered insulin release, there are two other
possibilities: the hypoglycemic episodes89, 90 in
PMT-C could trigger aldosterone secretion via ACTH
release63 or cause water retention via vasopressin
release.21
Lipids. PMTS patients derive 40% of their calories
from dietary lipids, consuming twice as much animal fats as
vegetable oils.52 The dietary goals for these patients
are a decrease to 30% of calories from fat and a
saturated:unsaturated ratio of 0.5, with monounsaturates and
polyunsaturates equally distributed (Table I). The
monounsaturates and polyunsaturates should come predominantly
from vegetable oils in order to emphasize oleic,
cis-linoleic and a-linolenic acid. Good sources of these
unsaturated fatty acids are, respectively, olive, safflower and
linseed oil. The ingestion of animal fats and hydrogenated
vegetable oils should be curtailed in order to minimize the in
take of arachidonic acid, saturated fatty acids and
transunsaturated fatty acids.
Explaining the rationale for the above recommendations
requires a review of the role of certain essential fatty acids as
precursors of prostaglandins84 and other compounds
that have important physiologic effects (Figure 1). Arachidonic
acid, present in animal fats, is the precursor of the series 2
prostaglandins, PGE2 and PGF2a;
the leukotrienes; and other compounds (Figure 1). Besides causing
inflammation of the breast and other tissues, the leukotrienes,
in minute amounts, elicit severe bronchospasm in
humans128 and could play an important role in the
premenstrual exacerbation of asthma.52
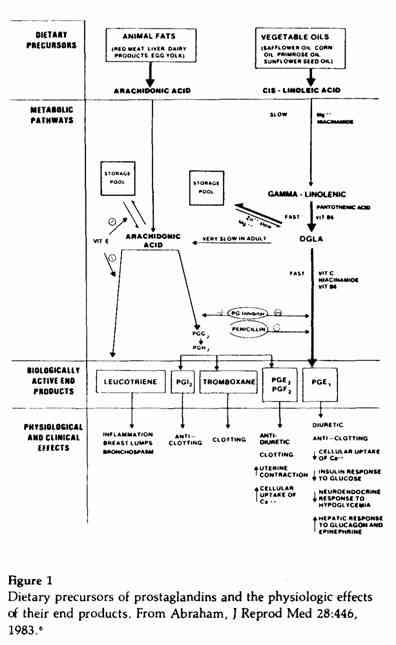
Cis-linoleic acid, present in most vegetable oils in
large quantities, is the precursor of the series 1
prostaglandins, PGE1 and PGF1α. As we
previously reported, the series 1 prostaglandins have beneficial
effects and series 2 have deleterious effects on PMTS
symptoms.6 The conversion of
cis-linoleic acid to arachidonic acid is very limited in
adult humans.27 The yield of series 2 prostaglandins
from arachidonic acid is 71%, whereas that of series 1
prostaglandins from cis-linoleic acid is
<5%.27 The fatty acid dihomogammalinolenic acid
(DGLA) is not available in the diet in significant quantities but
is derived from the metabolism of cis-linoleic acid with
the help of magnesium,28 niacinamide and vitamin
B6 (Figure 1). The yield of series 1 prostaglandins
from DGLA is 68%,27 therefore equivalent to the yield
of series 2 prostaglandins from arachidonic acid. For the above
reasons, the dietary ratio of cis-linoleic to
arachidonic acid must be very high (at least 100:1) in order to
maintain the proper balance between the series 1 and 2
prostaglandins. Intake of saturated and hydrogenated transfatty
acid blocks the conversion of cis-linoleic acid to
DGLA.75 Margarine, for example, is 10-65% transfatty
acid and is consumed in large amount by young U.S.
women.123 This dietary pattern would be detrimental to
the synthesis of series 1 prostaglandins. Limiting the intake of
these fats would increase the yield of series 1 prostaglandins
from cis-linoleic acid.70
Oleic acid is preferentially oxidized and used as a source of
calories as compared to cis-linoleic acid. Therefore,
oleic acid has a sparing effect on linoleic acid, which then can
be utilized as a precursor of series 1 prostaglandins. The fatty
acid a-linolenic acid is the precursor of series 3 prostaglandins
and eicosapentanoic acid (EPA) and has a modulating effect on the
synthesis of series 1 prostaglandins.83 The fatty acid
EPA suppresses the synthesis and release of arachidonic acid and
its further metabolism to the series 2 prostaglandins and
leukotrienes.27, 107
Both arachidonic acid and DGLA are stored in the cell membrane
as phospholipids.46 The fatty acid composition of cell
membranes, the main sources of prostaglandin precursors, change
within one week to reflect the fatty acid content of the
diet.46 Therefore, the effect of dietary modification
on prostaglandin synthesis is relatively rapid. The enzyme
phospholipase A2 releases these fatty acids for prostaglandin
synthesis.77 The physiologic and clinical effects of
phospholipase A2 stimulation would depend on the type of fatty
acids present in the phospholipids of the cell membrane and also
on the fatty acid composition of the diet consumed during the
previous week. Since phospholipase A2 activity is influenced by
zinc, magnesium and vitamin E,6,
39, 58, 99 the clinical effects of these micronutrients
would depend on the diet of PMTS patients consuming these
micronutrients. Besides its effect on phospholipase A2, vitamin E
blocks lypoxygenase activity and therefore suppresses leukotriene
formation.59
Another mechanism by which fatty acids influence PMTS-related
symptomatology is by their effects on estrogen metabolism. A
recent study compared blood and fecal estrogen levels in 10 white
and 12 east Asian premenopausal women.57 The white
women consumed 40% of their calories as fats, whereas only 22% of
the calories consumed by the Asian women came from fats. The
Asians consumed twice as much unsaturated as saturated fatty
acids, where as the whites ingested equal amounts of saturated
and unsaturated fats. The fecal estrogen level was significantly
higher and blood estrogen level significantly lower in the Asian
women. Since elevated peripheral estrogens are associated with
PMT-A symptoms,5-7 decreasing the intake of
fats may improve PMT-A symptoms. The authors57 found
no correlation between blood estrogens and polyunsaturates but a
highly significant and positive correlation between blood
estrogen levels and saturated fats. Therefore, the emphasis
should be on increasing the unsaturated:saturated ratio while
lowering the total fats consumed.
Proteins. As a group, PMTS patients consume 30% more
proteins than normal women do.55 There is a
significant and positive correlation between the consumption of
proteins and dairy products on the one hand and the severity of
PMT-A symptomatology on the other.12 In 39 PMTS
patients evaluated, the daily consumption (of protein, expressed
as percentage of Recommended Daily Allowance (RDA), and dairy
products, expressed as servings, was, respectively: no PMT-A =
140±15 and 1.33±0.41, moderate PMT-A =
239±28 and 2.8±0.5, and severe PMT-A =
376±59 and 5.7±0.88.12 The PMT-A
patients consumed three times more animal protein than vegetable
protein, whereas 14 normal women consumed on the average twice as
much vegetable protein as animal protein. However, both normal
women and PMTS patients consumed protein much in excess of the
RDA. Daily protein intake in the U.S. has been fairly constant
over the past 70 years, roughly 200% of the RDA, but the sources
have changed. The percentage of protein from vegetables decreased
from 48% in 1909 to 31% in 1970, while the percentage of protein
from animal sources, including dairy products, increased from 52%
in 1909 to 69% in 1970.102
Excessive intake of animal protein stimulates
prolactin,31 insulin67 and luteinizing
hormone (LH) secretions.66 Elevated prolactin causes
luteolysis, predisposing to PMT-A symptoms.5
Hyperinsulinism increases glucose tolerance and predisposes to
PMTC.6 Elevated LH levels increase the ovarian
secretion of androgens,121 which suppress estrogen
synthesis and predispose to PMT-D.4,7
Recent studies have suggested a significant effect of animal
proteins on the length of the menstrual cycle.65,
66 Women who ate a vegetarian (meatless) diet had a shorter
follicular phase and shorter menstrual cycle than when they ate a
daily meat supplement, with a mean cycle length of 27 and 29
days, respectively, for the vegetarian and meat-containing
diets.66 The basal LH levels were higher during the
follicular and luteal phases of the menstrual cycle when the
subjects ate a meat-containing diet. Isocaloric protein
supplementation with soybean proteins, instead of meat, failed to
modify the gonadotropin levels or the duration of the menstrual
cycle. Women who ate a meat-containing diet had higher
plasma-free tryptophan levels throughout the menstrual cycle than
when they ate a soybean protein supplement.53 Since
plasma free tryptophan is an active precursor of CNS serotonin
levels53 and CNS serotonin has a stimulating effect on
LH release,126 the increased CNS serotonin level
following meat consumption could explain the effects of meat
consumption on the menstrual cycle.
When two groups of healthy, menstruating women were placed on
a diet of 1,000 calories per day for six weeks, with one group on
a vegetarian diet and the other on a meat-containing diet, daily
mood ratings on a visual analog scale were significantly higher
in the women eating the meat-containing diet.105
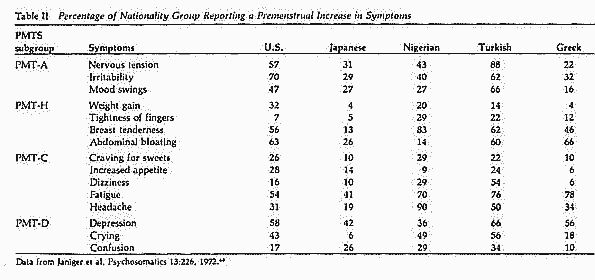
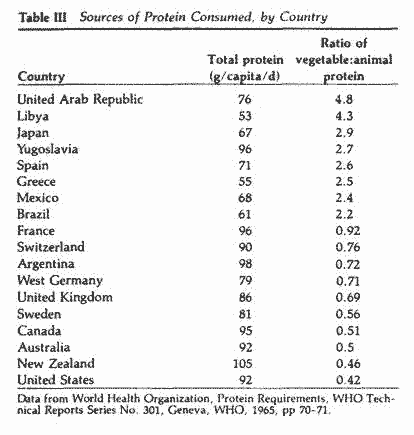
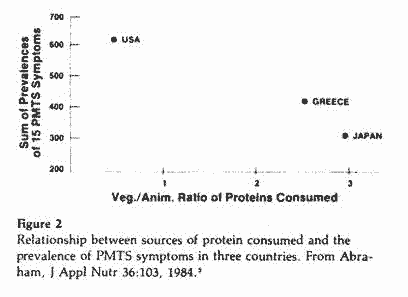
Based on data obtained by Janiger68 regarding the
prevalence of PMTS symptoms in the U.S., Japan and Greece (Table
II) and data from the World Health Organization on the sources of
proteins consumed by various nationalities (Table III), we
compiled and plotted these two variables on x-y axes for three
countries (Figure 2). The data, although limited in number,
suggest an inverse relationship between the prevalence of PMTS
symptoms and the vegetable:animal ratio of proteins consumed. If
this relationship is valid, one would expect to find a low
prevalence of PMTS in countries consuming proteins with a
vegetable:animal ratio >2 and a high prevalence in countries
where the ratio is <1.
Our recommendation is to lower total protein in take from 20%
to 15% of calories consumed, with a vegetable:animal ratio of
2.
Micronutrients
Although dietary surveys give an accurate index of the
macronutrient intake, that is not the case for micronutrients
since soil characteristics, methods of farming and processing
affect the micronutrient con tent of foods. For this reason,
supplementation of micronutrients is often required to increase
the nutrient density of the food consumed.
A dietary survey of normal women and PMTS
patients55 revealed that only two of the normal women
were not ingesting nutritional supplements and that these two
women were on a purely vegetarian diet at the time of the study.
In comparison, 33 of the 39 PMTS patients did not consume
nutritional supplements on a regular basis. The intake of B
vitamins, iron, zinc and manganese was significantly higher in
the normal women.
Vitamins. Some vitamins have been tested in PMTS
patients, in open trials and under controlled
conditions.104 Vitamin A, at daily doses of
100,000-300,000 IU for the last two weeks of the menstrual cycle,
improved PMT-H symptoms in 87-93% of the cases but was less
effective for PMT-A symptoms.17,26 The dosages of
vitamin A found to be useful in PMT-H averaged 50,000-150,000 IU
daily.17, 26, 104 The lowest dosage of vitamin A
reported to cause toxicity in adults is 40,000 IU taken daily for
many years.118, 48, 92 The recommended dosages of
vitamin A in PMT-H patients are potentially in the toxic range.
Zinc increases the mobilization of vitamin A from the liver, and
vitamin E prevents the oxidation of vitamin A.1
Therefore, these two nutrients may lower the effective dose of
vitamin A.
Vitamin E, at a daily dosage of 150 IU, improves PMT-A
symptoms but has no effect on PMT-H, PMT-C or PMT-D. At a daily
dosage of 600 IU it worsens PMT-A symptoms but improves PMT-C and
PMT-D symptoms.80 Since selenium has synergistic
effects with vitamin E,119 selenium supplementation
may lower the effective dose of vitamin E in PMTS patients.
In an open trial, vitamin B6 at a daily dosage of
40-100 mg was found effective in 50-60% of 70 PMTS
patients.74 In two control studies in which dosages of
100-500mg of B6 were used,11, 131 response
rates of 82-84% were observed, and the placebo effect in both
studies was significantly lower than the response to the
vitamin.
Since vitamin C decreases the estrogen clearance
rate29 and increases the biologic activity of
estrogens, megadoses may be useful in PMT-D patients.
Minerals. PMTS patients consume a daily average of
100 mg of magnesium less and 200% more sodium than do normal
women.55 The mean daily intakes of potassium and
calcium have not been found to be significantly different between
normal women and PMTS patients.
Low red cell magnesium levels have been found in PMTS
patients.2, 12 In an open trial of 192 PMTS patients,
magnesium supplementation during the week preceding menses
significantly improved nervous tension, mastalgia and weight gain
in 89-96% of the patients.96
PMT-A patients consume excessive amounts of calcium, mainly
from dairy products.2 Since calcium interferes with
the absorption and utilization of magnesium and other
nutrients6, 130 and the main source of calcium in PMTS
patients is dairy products, our dietary goal is to lower the
intake of dairy products and increase the magnesium and potassium
intake from vegetable sources. Limitation, but not restriction,
of sodium is also advised.6
Trace Elements. Normal women consume twice as much
iron and zinc and four times as much manganese as PMTS patients
do. The intake of copper, selenium and chromium is not different
between the two groups.55 So far there has been no
published study on the effect of selected trace elements on PMTS
symptoms.
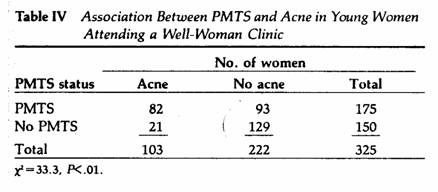
We observed a significant and positive correlation between
PMTS and premenstrual acne in 325 young women (X, M±SE =
25±2.5 years) attending a well-woman clinic (Table IV).
Zinc deficiency has been found in acne patients,106
and zinc supplementation improved the acne score under
double-blind conditions. PMTS patients with acne may benefit from
zinc supplementation. At a daily dosage of 50 mg, zinc suppresses
prolactin levels in hyperprolactinemic women without pituitary
tumors.113 Since elevated prolactin levels suppress P
secretion by the ovary104 and low P predisposes to
PMT-A symptoms,7 zinc supplementation may be indicated
in PMT-A patients with elevated prolactin not due to pituitary
tumors. Increased urinary chromium excretion has been
demonstrated in women following a glucose load.16
However, daily supplementation with 200 µg of trivalent
chromium prevented glucose-stimulated chromium excretion. Since
PMT-C patients consume excess amounts of refined
sugar,55 chromium supplementation may be of value.
Implementation of the Nutritional Program
After ruling out serious medical problems, implementation of
the nutritional program is warranted for a period of at least
three months: our previous experience indicated that the best
responses in PMTS patients were observed between three and six
months on the program.49, 54
The assistance of a dietician or nutritionist is needed to
formulate a menu appropriate in macronutrient content and
proportions (Table I). Food allergies and preferences are
important considerations in pre paring such a menu. In addition
to the recommendations in Table I, patients should be advised
that tobacco smoking suppresses estrogen
levels,20, 82 predisposing to
PMT-D,3 premature menopause69 and
osteoporosis.44 For these reasons, patients should be
advised against tobacco inhalation or chewing.
Methylxanthine-containing foods should be limited because of
their deleterious effects on breast cysts and mastalgia.86
,87 Alcohol inhibits gluconeogenesis and promotes a
distinct fall in plasma glucose,108 thereby
predisposing to PMT-C symptoms. Alcohol intake should be limited
to 5% or less of the calories consumed. We also recommend regular
and moderate exercise in the form of a fast walk for three to
five miles, five days a week.
For some of the micronutrients, we favor the use of
supplements in order to guarantee a minimum intake and also to
increase the nutrient density of the foods consumed.
As previously discussed,3,6 several micronutrients
are required for the synthesis of prostaglandins from essential
fatty acids and for the synthesis of CNS biogenic amines and
neuropeptides from amino acids. A deficiency in some
micronutrients may have a profound effect on the synthesis of
these neuroactive substances. For example, iron deficiency
decreases the synthesis of tyrosine from phenylalanine by
50%.78 Tyrosine is an important precursor of CNS
cathecolamines109 (vide infra), such as
dopamine, in which PMT-H patients are deficient.76 A
mild copper deficiency has a significant effect on brain levels
of endorphins, enkephalins and cathecolamines23
(vide infra).
Single-nutrient supplementation, although useful in PMTS
patients when tested for a few months only,11,
80, 96, 130 may not be effective and safe when administered
chronically.3
On the basis of published information regarding the role of
certain micronutrients in PMTS-related symptoms, one of us
(G.E.A.) formulated a nutritional supplement (Optivite, hereafter
referred to as the PMTS supplement) high in vitamin B6
and magnesium and also containing other known micronutrients in
quantities and proportions to meet or exceed the RDAs for these
nutrients.103
The nutritional program consists of the guidelines detailed in
Table I for macronutrients and administration of micronutrients
orally (PMTS supplement). The dosages of micronutrients are
adjusted within safe limits, according to the clinical responses
obtained and side effects experienced by the patients.
The nutrients that are potentially toxic at the dosages used
in the PMTS supplement are vitamin A1 and vitamin
B6.40, 100, 112 The maximum recommended
daily dosage of the PMTS supplement is 12 tablets containing
25,000 IU of vitamin A and 600 mg of vitamin B6.
Daily dosages of retinyl palmitate (vitamin A) ranging from
10,000 to 36,000 IU for a period of six months increase serum
vitamin A levels significantly but do not place them in the toxic
range.125 The supplement used in our program contains
25,000 IU of retinyl palmitate in 12 tablets, the maximum
recommended daily dosage. Retinyl palmitate is preferred over
carotene as a source of vitamin A because of serious side effects
reported with hypercarotenemia —hypothalamic amenorrhea,
leukopenia and neutropenia.73, 122
Vitamin B6 administration at daily dosages of
200—6,000 mg is associated with peripheral neuropathy in
some women.99, 112 We have observed that when vitamin
B6 is given alone (without other supplements), some
women tend to develop B6 tolerance within six months
and require an increased B6 dosage to produce the same
symptomatic relief. We therefore do not recommend megadosages of
vitamin B6 without the concomitant use of other
important micronutrients.
Pyridoxine, the form of vitamin B6 used in the
supplement, is biologically inactive. Two enzymatic steps are
required for the conversion of pyridoxine to pyridoxal phosphate
(PLP), the active form: phosphorilation of pyridoxine, which
requires magnesium, and oxidation of pyridoxine phosphate, which
requires riboflavin. The alternate pathway is oxidation of
pyridoxine to pyridoxic acid, which is excreted in the
urine.3 This alternate pathway is used when there is a
block in the conversion of pyridoxine to PLP. If pyridoxine is
the toxic form of vitamin B6, inefficient metabolism
to PLP could be an important factor in B6 toxicity. We
recently found an increased excretion of pyridoxic acid during
the luteal phase of the menstrual cycle,1 suggesting a
block in the conversion of pyridoxine to PLP during that
phase.
Aldosterone increases the urinary excretion of magnesium and
augments the formation of flavin nucleotides from
riboflavin.3 Aldosterone levels are elevated during
the luteal phase.3 Therefore, the hyperaldosteronism
of the luteal phase could explain the block in the conversion of
pyridoxine to PLP, due to depletion of magnesium and riboflavin.
Since progesterone increases aldosterone
secretion,97, 117 progesterone
administration could predispose to vitamin B6
neurotoxicity. It is of interest in this regard that Dalton
reported the largest series of B6 neurotoxicity to
occur with a dosage of B6 as low as 50 mg per
day.41 She prescribes extremely large dosages of
progesterone to her PMTS patients.40
To minimize the side effects of vitamin B6 therapy,
other micronutrients, mainly magnesium and other B vitamins,
should be given together with vitamin B6. The dosage
of the micronutrients should be adjusted every three months in
order to use the minimum effective dosage.
Our nutritional program for PMTS has been recommended by
physicians nationwide for several years. With an estimated
100,000 users, not a single case of hypervitaminosis A or
B6 has been reported, due in part to the fact that the
dosage of the PMTS supplement can be decreased within six months
of use since the severity of PMTS symptoms improves significantly
within that time.34, 49, 54
Effect of Nutritional Program on PMTS
Symptomatology
The effects of the nutritional program on PMTS symptoms have
been studied in open trials and con trolled studies.
Open trials are useful in assessing minimum effective dosages
and possible side effects. In two uncontrolled studies involving
47 PMTS patients who were on the program for one to six months,
the best responses were observed at a daily dosage of six
tablets. Some patients required an increased dosage
premenstrually, ingesting up to 12 tablets daily during the seven
to ten days of the menstrual cycle, mainly when the MSQ score for
the week before was > 30.49, 54 Symptom scores were
lowest after three to six months on this regimen.
Gastrointestinal side effects were observed occasionally at
dosages greater than six tablets daily.54
In another open trial of the nutritional program, we assessed
the importance of diet alone, PMTS supplementation alone and a
combination of diet and PMTS supplementation in the management of
PMTS. Four groups of patients were studied, with in formed
consent, and each completed the MSQ for one control and three
treatment cycles. Group I received no treatment except for a
one-a-day vitamin. Patients with the lowest MSQ scores were
placed in that group. Group II patients were only advised about
the dietary recommendations, and no supplement was given. Group
III patients received the PMTS supplement only at a daily dosage
of six tablets, but no dietary recommendations were made. Group
IV received both the PMTS supplement and dietary recommendations.
Patients with the highest MSQ scores were placed in groups III
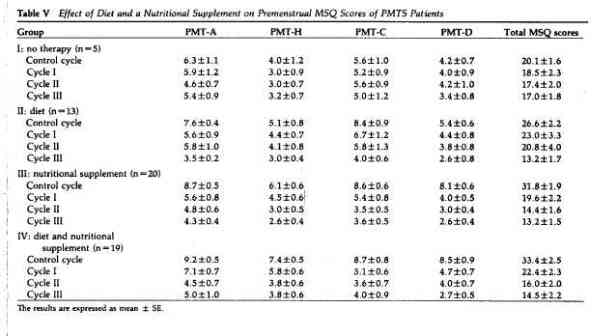
and IV. Following three months on the one-a-day vitamins, the
mean premenstrual MSQ scores were not significantly different
from the mean scores in the control cycle (group I, Table V). The
MSQ scores decreased more rapidly with the PMTS supplement alone
than with diet alone. After three months, the differences between
the pretreatment and posttreatment MSQ scores were significantly
greater with the PMTS supplement than with diet alone
(P< .05). When the data for each PMTS subgroup were
evaluated, there was no significant difference between the
effects of diet alone and PMTS supplementation alone on PMT-A and
PMT-C symptoms. However, the PMTS supplement alone had a
significantly greater effect (P< .01) than diet alone
on PMT-H and PMT-D symptoms. Diet plus the PMTS supplement (group
IV) gave results similar to those of the PMTS supplement alone.
This study demonstrated that the carefully formulated nutritional
supplement, tailored to PMTS patients, was effective in lowering
PMTS symptomatology whereas a one-a-day vitamin had no
significant effect. No side effects were reported.
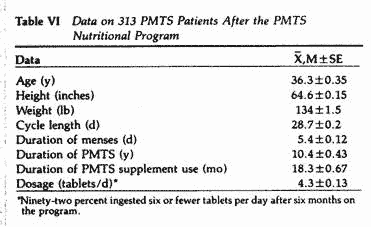
The impact of PMTS on the social, familial and work
performance of 313 women suffering from PMTS was self-assessed
using a postal survey. The subjects were also asked to complete
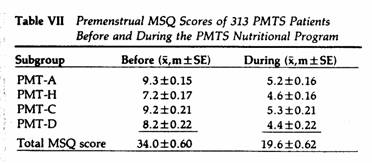
the MSQ. Following 2-60 months on the nutritional program, the
above parameters were again evaluated (Tables VI and VII). When
the degree of impairment caused by PMTS on social, familial and
work performance was compared with the premenstrual MSQ scores,
there was a significant (P< .01-.005) and positive
correlation between the MSQ scores and the degree of interference
on all three aspects of performance evaluated (Table VIII).
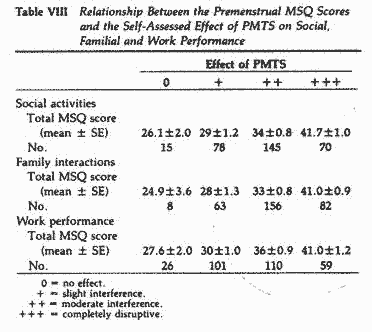
Following the nutritional program, there was a significant
(P<.01-.005) and positive correlation between the
initial MSQ scores and the degree of improvement in performance
in all three parameters. There was a significant (P<
.05) and negative correlation between the posttreatment MSQ
scores and the beneficial effect of the program on performance in
these three parameters (Table IX).

The subjects who assessed PMTS as having the most disruptive
effect on their lives and who had the highest MSQ scores prior to
the nutritional program and the lowest MSQ scores while on the
program reported the greatest improvement, by self-assessment, in
performance at home, at work and during social activities
following dietary modification and nutritional supplementation.
From the data in Table IX it is clear that the pre-post
differences in MSQ scores have a positive relationship with
degree of improvement observed: with a decrease in MSQ scores
averaging 10 points or less, no effect or a slight improvement
was reported; with a 15-point drop, a moderate improvement; and
with 20 points, a marked improvement. Although the MSQ scores did
not detect subtle changes in the quality of life, the MSQ appears
to be a reliable index of the degree of disruption caused by PMTS
and of the beneficial effect of PMTS management on familial,
social and work performance as assessed by the PMTS
sufferers.
The mean daily intake of the PMTS supplement decreased over
time: 5.2 ± 0.36 tablets between 2 and 6 months, 4.8
± 0.6 tablets between 7 and 12 months, 4.3 ± 0.48
tablets between 13 and 24 months and 3.2 ± 0.34 tablets
over 24 months. Twenty-two subjects experienced side
effects while on the program: 20 had gastrointestinal symptoms,
and 2 experienced increased diuresis, which they considered and
unpleasant side effect. Such an increase in diuresis would
be expected in patients with PMTH symptoms following the program.
The gastrointestinal side effects were gastric upset and nausea
(13), loose stools (5) and constipation (2). The gastrointestinal
side effects could be controlled with dosage adjustments and
patient education on the importance of ingesting the PMTS
supplement with meals.
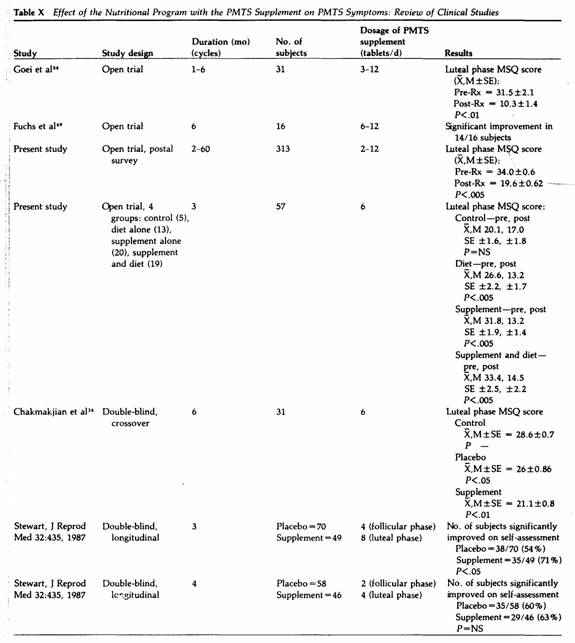
Based on three double-blind studies of 254 PMTS patients
(Table X), a dosage averaging six tablets daily lowered PMTS
symptoms significantly as compared to placebo, but at a daily
average dosage of three tablets, the effect of the PMTS
supplement was not greater than that of the placebo. These
studies were performed during a three- to four-month period, and
at present we do not have controlled studies over a prolonged
period to assess the minimum effective dosage over time. No side
effect was reported with a daily dosage averaging up to six
tablets in studies performed in the U.S., but in the two
controlled studies by Stewart on British women (Table X), some
10% of the subjects ingesting the PMTS supplement at a dosage of
three to six tablets daily experienced mild headache and
diarrhea.
Biochemical and Endocrine Effects of the Nutritional
Program
The effect of the nutritional program on blood chemistry was
assessed in 16 PMTS patients with moderate to severe symptoms
ingesting 6-12 tablets of the PMTS supplement daily.49
Blood samples were obtained prior to treatment and after three
and six months on the program. There was a significant increase
in serum fasting glucose, albumin, alkaline phosphatase, SGOT,
SGPT and serum potassium. None of these parameters was above the
normal range, however. There was a nearly significant drop in
free bilirubin levels (P= .06). In six PMT-C patients
with low fasting glucose levels, the levels in creased to normal
following six months of nutritional therapy. Overall, improved
liver function tests were observed in women on the program.
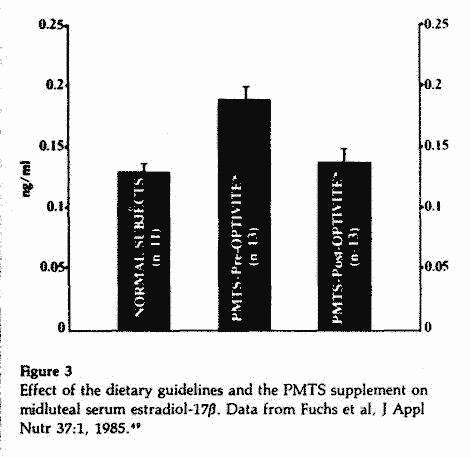
The effect of the nutritional program on the serum levels of
E2,P, aldosterone and dehydroepiandrosterone sulfate
(DHEA-S) during the midluteal phase was evaluated in 13 of the 16
PMTS patients.49 The mean E2 values were
higher than normal during the control cycles. Following three to
six months on the program, the mean serum E2 levels
decreased significantly and reached normal values (Figure 3).
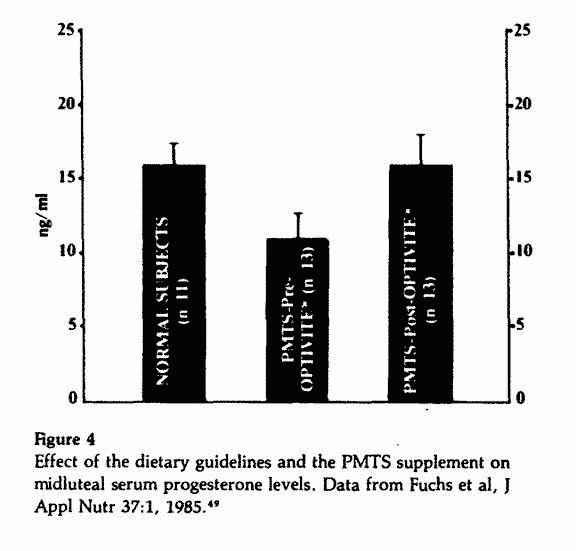
Midluteal P levels were lower than normal prior to initiation of
the nutritional regimen and increased significantly to normal
levels after three to six months (Figure 4). There was a
nonsignificant drop in serum aldosterone levels and no
significant change in DHEA-S levels following three to six months
of nutritional therapy.49
Management of Nonresponders
PMTS patients should be reevaluated as nonresponders in the
nutritional program if, after three to six months of
implementation, there is a less-than-moderate improvement in PMTS
symptoms according to the MSQ and MSD scores (a decrease of
<15 points in premenstrual MSQ scores from the control scores)
and a less-than-moderate improvement in the negative effects of
PMTS on the patient’s familial, social and work performance
according to self- assessment. A reasonable trial period for PMTS
patients with moderate symptoms (premenstrual MSQ score of <
30 points) is three months and for those with symptoms
(premenstrual MSQ score >30), six months.
The algorithm in Figure 5 outlines the management of
nonresponders in each PMTS subgroup.
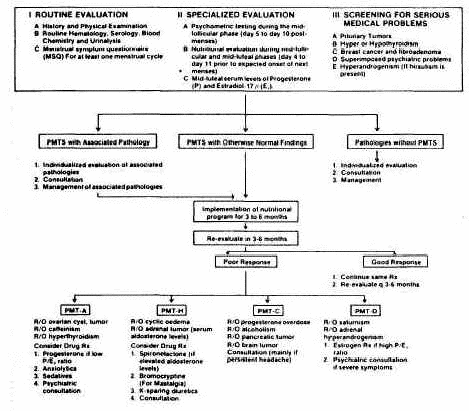
Figure 5
Evaluation and management of PMTS patients. First, identify
PMTS , and rule out serious medical problems; then, implement
the nutritional program; last, reevaluate and treat
nonresponders acccording to the results of the
reevaluation.
PMT-A
When symptoms resistant to the nutritional program are in the
PMT-.A subgroup, hyperthyroidism should be ruled out. The
possibility of caffeinism due to an increased intake of
caffeine-containing substances during the luteal phase should be
assessed. If serum P:E2 ratio is low prior to and
following three to six months on the program, P administration
would be indicated using the smallest effective dose. We favor
oral micronized P in a sustained-release form over vaginal or
rectal suppositories, injections or implants because of better
patient compliance6 and because it is the only mode of
P administration shown to be effective in decreasing PMTS
symptomatology under controlled conditions.33, 45
If the low midluteal P:E2 ratio is due to markedly
elevated E2 levels (>300 pg/mL of serum), it is
important to rule out ovarian tumors and cysts and other causes
of increased E2 production. Potential causes of
hyperestrogenemia in PMT-A patients are: (1) an increased
production rate (increased ovarian secretion rate [ cyst,
polycystic ovary syndrome, estrogen secreting ovarian tumors] or
increased peripheral aromatization of androgens by adipose tissue
[obesity]) and (2) a decreased clearance rate (decreased hepatic
clearance from decreased blood flow [ vascular diseases,
strenuous exercises] or decreased liver function, such as organic
diseases [ cyrrhosis] and decreased enzymatic activity, including
glucuronyl transferase and sulfokinase] or decreased intestinal
clearance (decreased binding of estrogens in the intestinal
tract, such as from a low-fiber diet or increased hydrolysis of
conjugated estrogens by intestinal flora, such as from a high
intake of animal fats]).3 E2 elevation from
an increased E2 production rate does not respond well
to nutritional therapy alone. A weight loss program is
indicated in obese patients in order to decrease the
aromatization of androgens by adipose tissue. Psychiatric
consultation should be obtained if the above measures fail.
PMT-H
When symptoms not responding to nutritional therapy belong to
the PMT-H subgroup, a careful charting of them over a period of
one or more menstrual cycles should be performed to rule out
idiopathic cyclic edema,74 in which the cyclicity of
the symptoms is not related to the menstrual cycle.
A potassium-sparing diuretic may be tried for one to three
cycles.33 Bromocriptine may be effective for
breast-related symptoms. If in doubt about the possible etiology,
the primary care physician should obtain an internal medicine
consultation. Midfollicular and midluteal serum aldosterone
levels may be used as an index of zona glomerulosa function and
to rule out aldosterone-secreting adrenal tumors.
PMT-C
In PMT-C nonresponders, alcoholism should be ruled out by a
careful reevaluation of life-style and alcohol
intake.6 Large doses of P may aggravate PMT-C because
of P’s hypoglycemic effects.6 Some PMT-C
patients may be under the care of several clinicians, receiving
different medications, including large doses of P. If this is the
case, the P should be discontinued or the dose decreased to 300
mg a day, preferably using the oral form.
If headache is the predominant symptom, a neurologic
consultation is indicated to rule out brain tumors.
PMT-D
If the midluteal P:E2 ratio is high due to low
E2 levels, both prior to and following three to six
months on the program, estrogen administration at a low dosage
during the luteal phase is warranted in PMT-D
nonresponders.7 In the presence of hypotyrosinemia,
3-6 g of L-tyrosine in the morning may be effective.5,
6 In cases of insomnia, 0.5-1.0 g of L-tryptophan at bed
time may be of value.5, 35 If hirsutism is present,
evaluation of hyperandrogenism of the adrenal and/or ovary is
indicated.13 Saturnism should be ruled out by a
careful evaluation of lead exposure at home, at work and from the
environment6,60; if a positive history is obtained,
blood and urine lead and red cell protoporphyrin levels are
indicated. Psychiatric consultation should be obtained in
patients with severe PMT-D.
Acknowledgments
The authors acknowledge the assistance of Kim Irvine, Dianne
Tartaglini and Harriet Porter in the implementation and
compilation of data from some of the studies described. The
assistance of Wayne Dederick in statistical evaluation is also
acknowledged.
References
1. Abraham GE: Bioavailability of selected nutrients from a
dietary supplement, Optivite for Women. J Appl Nutr 37:61,
1985
2. Abraham GE: Magnesium deficiency in premenstrual tension,
Magnesium Bull 4:68, 1982
3. Abraham GE: Management of the premenstrual tension
syndromes: Rationale for a nutritional approach. In A
Year in Nutritional Medicine. Edited by J Bland. New Canaan, CT,
Keats Publishing, 1986, pp 125-166
4. Abraham GE: The normal menstrual cycle. In
Endocrine Causes of Menstrual Disorders. Edited by JR Givens.
Chicago, Year Book Medical Publishers, 1978, pp 15-44
5. Abraham GE: Nutrition and the premenstrual tension
syndromes. J Appl Nutr 36:103, 1984
6. Abraham GE: Nutritional factors in the etiology of the
premenstrual tension syndromes. J Reprod Med 28:446, 1983
7. Abraham GE: Premenstrual tension. Curr Prob Obstet Gynecol
3:5, 1980
8. Abraham GE: The premenstrual tension syndrome. In
Contemporary Obstetric and Gynecologic Nursing. Third volume.
Edited by LK McNall. St Louis, CV Mosby. 1980. pp170-184
9. Abraham S, Beaumont F, Argall W, et al: Nutrient intake and
the menstrual cycle. Aust NZ J Med 11:210, 1981
10. Abraham GE, Eisner CW, Lucas LA: Hormonal and behavioral
changes during the menstrual cycle. Senologia 3:33, 1978
11. Abraham GE, Hargrove JT: Effect of vitamin B on
premenstrual symptomatology in women with premenstrual tension
syndrome: A double-blind crossover study. Infertility 3:155,
1980
12. Abraham GE, Lubran MM: Serum and red cell magnesium levels
in patients with premenstrual tension. Am J Clin Nutr 34:2364,
1981
13. Abraham GE, Maroulis GB, Boyers SF, et al: Dexamethasone
suppression test in the management of hyperandrogenized patients.
Obstet Gynecol 57:155, 1981
14. Abraham GE, Maroulis GB, Marshall JR: Evaluation of
ovulation and corpus luteum function using measurements of plasma
progesterone. Obstet Gynecol 44:522, 1974
15. Aldercrutz H, Fotsis T, Heikkinen R, et al: Excretion of
the lignans enterolactone and enterolactone and enterodiol and of
equol in omnivorous and vegetarian postmenopausal
women and in women with breast cancer. Lancet 2:1295, 1982
16. Anderson RA, Polansky MM, Bryden NA, et al: Urinary
chromium excretion of human subjects: Effects of chromium
supplementation and glucose loading. Am J Clin Nutr 36:
1184, 1982
17. Argonz J, Albinzano C: Premenstrual tension treated with
vitamin A. J Clin Endocrinol 10:1579, 1950
18. Babb RR, Kieraldo JH: Cirrhosis due to hypervitaminosis A.
West J Med 128:244, 1978
19. Backstrom T, Mattsson B: Correlation of symptoms in
premenstrual tension to oestrogen and progesterone concentrations
in blood plasma. Neuropsychobiology 1:80, 1975
20. Barbieri RL, McShane PM, Ryan KJ: Constituents of
cigarette smoke inhibit human granulosa cell aromatase. Fertil
Steril 46:232, 1986
21. Baylis PH. Zerbe RL, Robertson GL: Arginine vasopressin
response to insulin-induced hypoglycemia in man. J Clin
Endocrinol Metab 53:935, 1981
22. Belfer ML, Shader RI, Carroll M, et al: Alcoholism in
women. Arch Gen Psychiatr 25:540, 1971
23. Bhathena SJ, Recant L, Voyles NR, et al: Decreased plasma
enkephalins in copper deficiency in man. Am J Clin Nutr 43: 42,
1986
24. Bickers W, Woods M: Premenstrual tension: Its relation to
abnormal water storage. N Engl J Med 245:453, 1951
25. Biskind MS. Biskind GR, Biskind LH: Nutritional deficiency
in the etiology of menorrhagia, metrorrhagia, cystic, mastitis,
and premenstrual tension. Surg Gynecol Obstet 78:49, 1944
26. Block E: The use of vitamin A in premenstrual tension.
Acta Obstet Gynecol Scand 39:586, 1960
27. Brenner RR: Metabolism of endogenous substrates by
microsomes. Drug Metab Rev 6:155, 1977
28. Brenner RR: The oxidative metabolism of unsaturated fatty
acids. Molec Cell Biochem 3:41, 1974
29. Briggs MH: Megadose vitamin C and metabolic effects of the
pill. Br Med J 283:1547, 1981
30. Brooks PC: Epidemiology and risk factors in breast cancer.
Reprod Med 27:670, 1982
31. Carson HE, Wasser HL, Levin SR, et al: Prolactin
stimulation by meals is related to protein content. J Clin
Endocrine Metab 57:334, 1983
32. Carstensen H, Backstrom T: Estrogen and progesterone in
plasma in relation to premenstrual tension. J Ster Biochem 5:
527, 1974
33. Chakmakjian ZH: A critical assessment of therapy for the
premenstrual tension syndrome. J Reprod Med 28:532, 1983
34. Chakmakjian ZH, Higgins CE, Abraham CE: The effect of a
nutritional supplement, Optivite for Women, on premenstrual
tension syndromes: II. Effect on symptomatology, using a double
blind cross-over design. J Appl Nutr 37:12, 1985
35. Coppen A, Shaw DM, Herzberg B, et al: Tryptophan in the
treatment of depression. Lancet 1:1178, 1967
36. Cowan LD, Gordis L, Tonascia JA, et al: Breast cancer
incidence in women with a history of progesterone deficiency. Am
J Epidemiology 114:209, 1981
37. Crapo PA, Reaven C, Olefsky J: Plasma glucose and insulin
responses to orally administered simple and complex
carbohydrates. Diabetes 25:741, 1976
38. Cummings JH: Nutritional implications of dietary fiber. Am
J Clin Nutr 31:S21, 1978
39. Cunnane SC: Differential regulation of essential fatty
acid metabolism to the prostaglandins: Possible basis for the
inter action of zinc and copper in biological systems. Prog Lipid
Res 21:73, 1982
40. Dalton K: The Premenstrual Syndrome and Progesterone
Therapy. Chicago, Year Book Medical Publishers, 1984
41. Dalton K: Pyridoxine overdose in premenstrual syndrome.
Lancet 1:1168, 1985
42. Danis RP, Newton N, Keith L: Pregnancy and alcohol. Curr
Prob Obstet Gynecol 4:5, 1981
43. Dalvit SP: The effect of the menstrual cycle on patterns
of food intake. Am J Clin Nutr 34:1811, 1981
44. Daniell HW: Osteoporosis of the slender smoker. Arch
Intern Med 136:298, 1976
45. Dennerstein L, Spencer-Gardner C, Gotts C, et al:
Progesterone and the premenstrual syndrome: A double blind
crossover trial. Br Med J 290:1617, 1985
46. Dorfman K: Dietary manipulation of prostaglandins with
special applications to heart disease. J Appl Nutr 37:108,
1985
47. Englyst HN, Cummings JH: Digestion of the polysaccharides
of some cereal foods in the human small intestine. Am J Clin Nutr
42:778, 1985
48. Farris WA, Erdman JW: Protracted hypervitaminosis A
following long-term, low-level intake. JAMA 247:1317, 1982
49. Fuchs M, Hakim M, Abraham GE: The effect of a nutritional
supplement, Optivite for Women, on premenstrual tension
syndromes: I. Effect on blood chemistry and serum steroid levels
during the midluteal phase. J Appl Nutr 37:1, 1985
50. Garnett ES, Cohen H, Nahmias C, et al: The role of
carbohydrate, renin and aldosterone in sodium retention during
and after total starvation. Metabolism 22:867, 1973
51. Gersing A, Bloom WL: Glucose stimulation of salt retention
in patients with aldosterone inhibition. Metabolism 11:329,
1962
52. Gibbs CJ, Coutts II, Lock R, et al: Premenstrual
exacerbation of asthma. Thorax 39:833, 1984
53. Glaecer BS, Maher TT, Wurtman RJ: Changes in brain levels
of acidic, basic and neutral aminoacids after consumption of
single meals containing various proportions of protein. Neurochem
41:1016, 1983
54. Goei CS, Abraham CE: Effect of a nutritional supplement,
Optivite. on symptoms of premenstrual tension. J Reprod Med
28:527, 1983
55. Goei CS, Ralston JL, Abraham CE: Dietary patterns of
patients with premenstrual tension. J Appl Nutr 34:4, 1982
56. Goldin BR, Adlercreutz H, Gorbach SL, et al: Estrogen
excretion patterns and plasma levels in vegetarian and omnivorous
women. N Engl J Med 307:1542, 1982
57. Goldin BR, Adlercreutz H, Gorbach SL, et al: The
relationship between estrogen levels and diets of Caucasian
American and Oriental immigrant women. Am J Clin Nutr 44:945,
1986
58. Guelix E, Rayssiguier Y: Activité lipolytique post
heparine chez le rat carence en magnesium. In Proc of
12th European Medical Association Congress on Magnesium, Paris,
1984, p 136
59. Gwebu ET, Trewyn RW: Vitamin E and the inhibition of
platelet lipoxygenase. Res Commun Chem Pathol Pharmacol 28:361,
1980
60. Hanson MA, Goei C, Abraham GE: Hair tissue concentration
of minerals, trace elements, and toxic metals in normal women and
patients with premenstrual tension syndromes. In Health
Effects and Interactions of Essential and Toxic Elements. Edited
by M Abdulla, BM Nair, RK Chandra. London, Pergamon Press, 1983,
pp 608-611
61. Hargrove JT, Abraham GE: The incidence of premenstrual
tension in a gynecologic clinic. J Reprod Med 27:721, 1982
62. Hargrove JT, Abraham GE: The ubiquitousness of
premenstrual tension in gynecologic practice. J Reprod Med 28:
435, 1983
63. Hata S. Kunita H, Okamota M: Aldosterone response to
hypoglycemia: Evidence of ACTH mediation. J Clin Endocrinol Metab
43:173, 1976
64. Hill P, Cohen L, Wynder EL, et al: Diet and endocrine
related cancer. Cancer 39:1820, 1977
65. Hill P. Garbaczewski L, Haley N, et al: Diet and
follicular development. Am J Clin Nutr 39:771, 1984
66. Hill PB, Garbaczewski L, Daynes C, et al: Gonadotrophin
release and meat consumption in vegetarian women. Am J Clin Nutr
43:37, 1986
67. Hoogwerf BJ, Lame DC, Greene E: Urine C-peptide and
creatinine (Jaffe Method) excretion in healthy young adults on
varied diets: Sustained effects of varied carbohydrate, protein
and meat content. Am J Clin Nutr 43:350, 1986
68. Janiger O Riffenburgh R, Kersh R: Cross cultural study of
premenstrual symptoms. Psychosomatics 13:226, 1972
69. Jick H, Porter J: Relation between smoking and age of
natural menopause. Lancet 1:1354, 1977
70. Jones PJH, Pencharz PB, Clandinin MT: Whole body oxidation
of dietary fatty acids: Implications for energy utilization. Am J
Clin Nutr 42:769, 1985
71. Kapala LP: Galactorrhea and thyrotoxicosis. Arch Intern
Med 144:2349, 1984
72. Kelsay JL, Behall KM. Prather ES: Effect of fiber from
fruits and vegetable on metabolic responses of human subjects: I.
Bowel transit time, number of defecations, fecal weight, urinary
excretions of energy and nitrogen and apparent digestibilities of
energy, nitrogen, and fat. Am J Clin Nutr 31: 1149, 1978
73. Kemmann E, Pasquale SA, Skaf R: Amenorrhea associated with
carotenemia. JAMA 249:926, 1983
74. Kerr GD: The management of the premenstrual syndrome. Curr
Med Res Opin 4:29, 1977
75. Kinsella JE, Bruckner G, Mai J, et al: Metabolism of
transfatty acids with emphasis on the effects of trans, trans
Octadecadienoate on lipid composition, essential fatty acid,
and prostaglandins: An overview. Am J Clin Nutr 34:2307,
1981
76. Kuchel O, Cuche JL, Hamet P. et al: Idiopathic edema: New
pathogenetic and therapeutic aspects. Mod Med Can 31:619,
1976
77. Kunze H, Vogt W: Significance of phospholipase A2 for
prostaglandin formation. Ann NY Acad Sci 180:123, 1971
78. Lehmann WD, Heinrich C: Impaired phenylalanine-tyrosine
conversion in patients with iron-deficiency anemia studied by a
L-(2H5)phenylalanine-loading test. Am J Clin Nutr 44:468,
1986
79. Liebowitz MR, Klein DF: Hysteroid dysphoria. Psychiatr
Clin North Am 2:555, 1979
80. London RS, Sundaram CS, Murphy L, et al: The effect of
α-tocopherol on premenstrual symptomatology: A double-
blind trial. J Am Coll Nutr 2:115, 1983
81. Lyon KE, Lyon MA: The premenstrual syndrome. J Reprod Med
29:705, 1984
82. MacMahon B, Trichopoulos D, Cole P, et al: Cigarette
smoking and urinary estrogens. N Engl J Med 307:1062, 1982
83. Marshall LA, Johnston PV: Modulation of tissue
prostaglandin synthesizing capacity by increased ratios of
dietary alpha linolenic acid to linoleic acid. Lipids 17:905,
1982
84. Mayes PA: Lipids. In Harper’s Review of
Biochemistry. Twentieth edition. Edited by DW Martin Jr. Los
Altos, CA, Lange Medical Publishers, 1985, pp 208-256
85. Michaelson C, Juhlin L, Vahiquist A: Effects of oral zinc
and vitamin A in acne. Arch Dermatol 113:31, 1977
86. Minton JP, Foecking MK, Webster DJ, et al: Caffeine,
cyclic nucleotides and breast disease. Surgery 86:105, 1979
87. Minton JP, Foecking MK, Webster DJ, et al: Responses of
fibrocystic disease to caffeine withdrawal and correlation of
cyclic nucleotides with breast disease. Am J Obstet Gynecol
135:157, 1979
88. Moos RH, Koppel BS, Melgen FT, et al: Fluctuations in
symptoms and moods during the menstrual cycle. J Psychosom Res
13:37, 1969
89. Morton JH: Premenstrual tension. Am J Obstet Gynecol
60:343, 1950
90. Morton JH: Symposium on premenstrual tension. Intern Rec
Med 166:463, 1953
91. Morton JH, Addition H, Addison C, et al: A clinical study
of premenstrual tension. Am J Obstet Gynecol 65:1182, 1953
92. Muenter MD, Perry HO, Ludwig J: Chronic vitamin A
intoxication in adults. Am J Med 50:129, 1971
93. Mukherjee C: Premenstrual tension: A critical study of the
syndrome. J Indian Med Assoc 24:82, 1954
94. Munday MR, Brush MC, Taylor RW: Correlations between
progesterone, oestradiol and aldosterone levels in the pre
menstrual syndrome. Clin Endocrinol 14:1, 1981
95. Munro HN: Recommended Dietary Allowances. Washing ton, DC,
National Academy of Sciences, 1980, pp 31-38
96. Nicholas A: Traitement du syndrome pré-menstruel et
de la dysmenorrhée par l’ion magnesium. In
First International Symposium on Magnesium Deficiency in Human
Pathology. Edited by J Durlach. Paris, Springer Verlag, 1973, pp
261-263
97. Oelkers W, Schoneshofer M, Blumel A: Effects of
progesterone and four synthetic progestagens on sodium balance
and the renin-aldosterone system in man. J Clin Endocrinol Metab
39:882, 1974
98. Owen OE, Kavel E, Owen RS; et al: A reappraisal of caloric
requirements in healthy women. Am J Clin Nutr 44:1, 1986
99. Panganamala RV, Cornwall DC: The effects of vitamin E on
arachidonic acid metabolism. Ann NY Acad Sci 396:376, 1982
100. Parry GJ, Brodesen DE: Sensory neuropathy with low dose
pyridoxine. Neurology 35:1466, 1985
101. Petrakis NL, King FB: Cytological abnormalities in nipple
aspirates of breast fluid in women with severe constipation.
Lancet 2:1203, 1981
102. Phipard EF: Protein and amino acids in diets. In
Improvement of Protein Nutritive. Edited by A Harper. Washington,
DC, National Academy of Sciences, 1974, pp 167-172
103. Physicians’ Desk Reference for Nonprescription
Drugs. Third edition. Oradell, NJ, Medical Economics, 1982
104. Piesse JW: Nutrition factors in the premenstrual
syndrome. Intl Clin Nutr Rev 4:54, 1984
105. Parke KM. Schreiber U, Laessle R, et al: Dieting
influences the menstrual cycle: Vegetarian versus no vegetarian
diet. Fertil Steril 46:1083, 1986
106. Pohit J, Saha KC, Pal B: Zinc status of acne vulgaris
patients. J Appl Nutr 37:18, 1985
107. Prescott SM: The effect of eicosapentaenoic acid on
leukotriene B. production by human neutrophils. J Biol Chem
260:1, 1984
108. Rabin D: Hypoglycemia: Physiologic and diagnostic
considerations. In Radioassay Systems in Clinical
Endocrinology. Edited by CE Abraham. New York, Marcel Dekker,
1981, pp 609-624
109. Rasmussen DD, Ishizuka B, Quigley ME, et al: Effects of
tyrosine and tryptophan ingestion on plasma catecholamine and
3,4-dihydroxyphenylacetic acid concentrations. J Clin Endocrinol
Metab 57:760, 1983
110. Rees L: The premenstrual tension syndrome and its
treatment. Br Med 1 1:1014, 1953
111. Schaller D: Fiber content and structure in foods. Am J
Clin Nutr 31:S99, 1978
112. Schaumberg H, Kaplan J, Windebank A: Sensory neuropathy
from pyridoxine abuse. N Engl J Med 309:445, 1983
113. Schumert Z, Linder N, Statter M: The effect of zinc in
human hyperprolactinemic state. In Proc of 32nd Annual
Meeting of the Society for Gynecologic Investigation, Scottsdale,
AZ, 1985, p 32
114. Schusdziarra V, Dangel C, Kilier M, et al: Effect of
solid and liquid carbohydrates upon postprandial pancreatic
endocrine function. J Clin Endocrinol Metab 53:15, 1981
115. Smith SL, Sauder C: Food cravings, depression, and
premenstrual problems. Psychosomat Med 31:281, 1969
116. Stieglitz EJ, Kimble ST: Premenstrual intoxication. Am J
Med Sci 218:616, 1949
117. Sundsfjord J: Plasma renin activity and aldosterone
excretion during prolonged progesterone administration. Acta
Endocrinol 67:483, 1971
118. Sutherland H, Stewart I: A critical analysis of the
premenstrual syndrome. Lancet 1:1180, 1965
119. Tappet AL: Vitamin E and selenium protection from in vivo
lipid peroxidation. In Micronutrient Interactions.
Edited by OA Levander, L Cheng. New York, New York Academy of
Sciences, 1980, pp 18-32
120. Tonks CM: Premenstrual Tension. Br J Psychiatr special
pub no 9, 1975
121. Tsang BK, Armstrong DT, Whitfield JF: Steroid
biosynthesis by isolated human ovarian follicular cells in vitro.
J Clin Endocrinol Metab 51:1407, 1980
122. Vakil DV. Ayiomamitis A, Nizami N, et al:
Hypercarotenemia: A case report and review of the literature.
Nutr Res 5: 911, 1985
123. Van den Reen MM, Craig-Schmidt MC, Weete JD, et al: Fats
in the diets of adolescent girls with emphasis on isomeric fatty
acids. Am J Clin Nutr 43:530, 1986
124. Van Soest PJ: Dietary fibers: Their definition and
nutritional properties. Am J Clin Nutr 31:S12, 1978
125. Wald NJ, Cuckle HS, Barlow RD, et al: The effect of
vitamin A supplementation on serum retinol and retinol binding
protein levels. Cancer Let 29:203, 1985
126. Walker RF, Wilson CA: Changes in hypothalamic serotonin
associated with amplification of LH surges by progesterone in
rats. Neuroendocrinology 37:200, 1983
127. Webb P: 24-hour energy expenditure and the menstrual
cycle. Am J Clin Nutr 44:614, 1986
128. Weiss JW, Drazen JM, McFadden ER Jr, et al: Airway
constriction in normal humans produced by inhalation of
leukotriene. J Am Med Assoc 249:2814, 1983
129. Wentz AC: Progesterone therapy of the inadequate luteal
phase. Curr Prob Obstet Gynecol 6:4, 1982
130. Wilgus HS Jr, Datton AR: Factors affecting manganese
utilization in the chicken. J Nutr 18:35, 1939
131. Williams MJ, Harris RI, Dean BC: Controlled trial of
pyrodoxine in the premenstrual syndrome. J Int Med Res 13:174.
1985
132. Wurtman RJ: Control of neurotransmitter synthesis by
precursor availability and food consumption. In Subcellular
Mechanisms in Reproductive Neuroendocrinology. Edited by F
Naftolin, KJ Ryan, IJ Davies. New York. Elsevier Scientific
Publishing. 1976, pp 149-166
From Optimox, Inc., Torrance, California, and the Student
Health Department, University of Colorado, Fort Collins.
Dr. Abraham is Medical Director, Optimox, Inc.
Dr. Rumley was Physician in Charge, Student Health Department,
University of Colorado, Fort Collins, and is now retired.
Address reprint requests to: Guy E. Abraham, 2720 Monterey
Street, Suite 406, Torrance, CA 90503.
This page was first uploaded to The Magnesium Web Site on July
19, 2002
http://www.mgwater.com/















