Journal of Internal Medicine 1999;
246: 373-378
Can one really measure magnesium deficiency using the
short-term magnesium loading test?
P. M. ROB1, K. DICK1, N.
BLEY1, T. SEYFERT1, CH. BRINCKMANN1, V.
HÖLLRIEGEL2, H. J. FRIEDRICH3, L.
DIBBELT4 & M. S. SEELIG5
From the 1Medical Department 1. Medizinische
Universität zu Lübeck; 2Institute of
Molecular Biology and Biochemistry, Freie Universität
Berlin; 3Institute of Medical Statistics, Medizinische
Universität zu Lübeck; 4Institute of
Clinical Chemistry, Medizinische Universität zu Lübeck,
Germany; 5University of North Carolina, Chapel Hill,
USA
Abstract. Rob PM, Dick K, Bley N, Seyfert T,
Brinckmann CH, Höllriegel V, Friedrich HJ, Dibbelt L, Seelig
MS (Medizinische Universität zu Lübeck, Freie
Universität Berlin, Germany and University of North
Carolina, Chapel Hill, USA). Can one really measure magnesium
deficiency using the short-term magnesium loading test? J
Intern Med 1999 246: 373-378.
Objective. To compare a 1-h-version of a
magnesium-loading-test (MLT) designed for outpatients in healthy
controls with the 8-h standard; to establish the test in patients
after renal transplantation prone to develop magnesium (Mg)
deficiency; to correlate femur Mg-concentration and percentage
retention of the given load.
Design. Comparison of mean values from
healthy controls with respective from the literature; a
prospective, randomized, controlled 4-month study; an
intra-individual correlation of Mg-serum values and loading-test
data with femur-Mg concentrations.
Setting. One centre study in a medical
university; outpatients from the transplant unit; inpatients from
the orthopedic unit.
Subjects. Twenty-four healthy controls aged
36.7 ± 7.4 years; 34 patients after renal transplantation
(46.5 ± 14.3 years); 41 patients with hip replacement
therapy (63.9 ± 18.6 years).
Intervention. Baseline Mg values were
measured by atomic absorption spectroscopy (AAS) in serum and
urine. An intravenous Mg load with 0.1 mmol Mgaspartate
hydrochloride per kilogram bodyweight was given during 1 h. In 24
h-urine, the amount of excreted Mg was measured by AAS and the
percentage retention of the given load calculated according to
the formula: 1 - [Mg 24 h-urine/Mg test dose] X 100. Femur Mg was
measured by AAS in a piece of the femur neck. Patients after
renal transplantation were randomized after the first Mg load to
either obtain daily 5 mmol Mg-aspartate hydrochloride per
kilogram bodyweight, or placebo. Four months later a second
loading-procedure was performed.
Main outcome measure. Serum Mg, percentage
retention of the given Mg load (%Ret) and femur Mg
concentration.
Results. Mean serum Mg values were within the
normal range. In controls, %Ret was -18 ± 21 and not
different from the literature. In the first MLT after renal
transplantation, %Ret was 47 ± 43. In patients under Mg
medication it decreased significantly to 16 ± 26, but was
58 ± 27 in the placebo group. Femur Mg concentration was
62.6 ± 20.9 mmol kg-1 dry substance and the
corresponding %Ret was 14 ± 28 with r = -
0.7093.
Conclusion. The short-term version of the MLT
is as good as the standard and was easily applied in outpatients.
The indication from the good correlation between bone-Mg and %Ret
and a marked decrease in %Ret in patients after Mg medication was
that one can really measure magnesium deficiency.
Keywords: magnesium deficiency, magnesium
loading test, magnesium retention.
Introduction
Magnesium (Mg) is involved in numerous biochemical and
physiological processes; its deficiency has been shown to play a
role in a variety of clinically important diseases [1]. It is
crucial to be able to detect magnesium deficiency [2-4]. However,
the most widely used test - serum magnesium - is not a reliable
index of the magnesium status. Only a minority of patients with
abnormal serum Mg levels is identified by physician initiated
order of the serum Mg level [5]. Hypomagnesemia, as reflected by
low serum Mg, indicates Mg deficiency, but intracellular Mg
depletion - involving many tissues - can exist despite normal
serum Mg levels [6, 7]. There is poor correlation between serum
Mg and tissue pools of Mg, except for the interstitial fluid [2].
Less than 1% of total body Mg is in the blood, and only 0.3% is
in the serum, yet most clinical data derive from serum sampling
and analysis [8]. Of the 1% of Mg that is in the blood, most is
in the cells and platelets, and analysis of Mg in erythrocytes,
lymphocytes, mono-nuclear blood cells and platelets have yielded
important data, as have muscle and bone biopsies. The techniques,
however, are too time consuming, too invasive and/or too
expensive for routine measurement [9, 10] and the results have
been conflicting [9, 11]. Furthermore, there is little evidence
for a dynamic equilibrium amongst body tissues [12]. The
magnesium-loading-test (MLT) was first used as a means to verify
Mg depletion induced in two healthy volunteers who had submitted
to dietary Mg deficiency on a diet that provided only 1.1 mEq of
Mg a day for three to four weeks [13]. The two young men retained
25 and 45% of the intravenously administered Mg at the end of the
study. The first clinical report showing the utility of the MLT
was in patients who experienced gastrointestinal Mg loss [14]. In
that study, the Mg was administered either intramuscularly or
intravenously, and retention of 20% or more was considered
evidence of deficiency. There has been growing interest amongst
cardiologists in detecting Mg deficiency by means of the MLT [15,
16]. As many as three quarters of 100 elderly patients with
congestive heart failure, hypertension, and/or diabetes mellitus
were found to be Mg deficient by the MLT [17]. In a larger study
of patients, predominantly with cardiovascular diseases, but with
representation of diabetics, alcoholics and some with
gastrointestinal disorders, who were given intravenous Mg loading
over 8 hours [18], the mean percentage retention of Mg ranged
between 22% and 54%. A reference range for Mg retention in 88
healthy men and women between 18 and 66 years of age, who were
given 30 mmol of Mg i.v. during 8 h and urine collection from
start of infusion for 24 h was given by Gullestad et al.
[4]. Their mean Mg retention was 6.3 ± - 10.3% and the
0.025 and 0.975 fractiles were -19.5% and 27.5% of the loading
dose, significantly less than the same investigative group found
in the patients population [18]. Because even 8 hours infusion of
the Mg load is unsuitably long for outpatients, we have
undertaken studies of 1-hour Mg infusion in healthy volunteers
[19] and in patients who had undergone renal transplantation with
(Mg wasting) immunosuppressive drug therapy [20]. Presented here
are additional details on those studies, with the addition of
comparative retention and bone Mg level data on hip replacement
patients with gastrointestinal disorders, who were given
intravenous Mg loading over 8 hours [18], the mean percentage
retention of Mg ranged between 22% and 54%. A reference range for
Mg retention in 88 healthy men and women between 18 and 66 years
of age, who were given 30 mmol of Mg i.v. during 8 h and urine
collection from start of infusion for 24 h was given by Gullestad
et al. [4]. Their mean Mg retention was 6.3 ± -
10.3% and the 0.025 and 0.975 fractiles were -19.5% and 27.5% of
the loading dose, significantly less than the same investigative
group found in the patients population [18]. Because even 8 hours
infusion of the Mg load is unsuitably long for outpatients, we
have undertaken studies of 1-hour Mg infusion in healthy
volunteers [19] and in patients who had undergone renal
transplantation with (Mg wasting) immunosuppressive drug therapy
[20]. Presented here are additional details on those studies,
with the addition of comparative retention and bone Mg level data
on hip replacement patients.
Subjects and methods
Study of the feasibility of a 1-hour infusion test load of Mg,
was undertaken to determine percentage retention - for assay of
the Mg status of three groups of test subjects - according to the
recommendations of the local ethics committee. All subjects gave
written informed consent. Group 1 comprised 24 healthy
individuals, who received no medication; Group 2 was made up of
34 patients 4 months after renal transplantation, who were being
immunosuppressed with ciclosporine, and who were prospectively
randomized after the first loading dose of Mg to receive: (i) 5
mg kg-1 bodyweight per day magnesium aspartate hydrochloride
(Magnesiocard; Verla Pharm, Tutzing, Germany) by mouth; or (ii)
placebo, for 4 months - after which the MLT was repeated. Group 3
was made up of 41 patients who received a MLT after hip
replacement and who had a 1-cm bone slice cut from the middle of
the removed femur neck, which after washing in cold NaCl 0.9% was
stored at -20 °C for analysis for bone Mg.
Loading-test procedure
The MLT was performed between 8:00 h and 10:00 h. Exclusion
criteria were AV-block, heart rate 5560 beats min-1, blood
pressure =110/ 70 mm mercury, pulmonary congestion or dyspnoe,
serum creatinine concentration >200 µmol L-1 and
proteinuria >500 mg day-1. After emptying the urinary bladder,
0.1 mmol Mg per kilogram bodyweight as the aspartate
hydrochloride (Magnesiocard; Verla Pharm) in 500 mL isotonic
saline were infused over 1 hour. Blood samples were drawn without
stasis 60 min after starting the infusion, to measure the peak
serum magnesium produced by the load. Blood pressure and
electrocardiogramms were recorded twice during the infusion.
Urine was collected in plastic bottles containing 15 mL 10% HC1,
from the start of the infusion for 24 h. The urine as measured to
the nearest of 50 mL, and an aliquot of the entire sample was
sent by outpatients to our laboratory by mail. Subjects were
informed of Mg-rich foods that were to be avoided during the
collection period. Mg retention was calculated from the 24-h
urinary Mg excretion and expressed as a percentage of the amount
of the Mg test dose according to the formula [17]:
I - [ Mg in 24 h urine/ Mg test dose] x 100
Dietary intake, fecal excretion and basal urinary magnesium
output were ignored. The freeze dried bone was analysed for Mg by
dissolving 0.2-0.5 g of the bone by heating with 10 mL 10 N HNO3.
The solution was given into a 100-mL beaker which was filled up
with aqua bidestillata. Then, 200 µL were taken and put
into 4 mL 0.1% La/0.1 N HC1 which was measured by atomic
absorption spectrophotometry [21] (AAS 1100; Perkin Elmer,
Lindau, Germany). Urine Mg was also determined by AAS. Data are
given as mean ± SD. Percent retention of the
magnesium-load is given as the median, and although it is
statistically not allowed to give the standard deviation in case
of percentage data, we did so according to the literature, for
better comparison. Statistical analysis was done by ANOVA,
Scheffe's test and Pearson's correlation. *Indicates a
statistical significant difference with a P = 0.05.
Results
Neither in healthy individual, nor in patients, was there
significant change in blood pressure, heart frequency, or
electrocardiogram during the MLT infusion. Some individuals
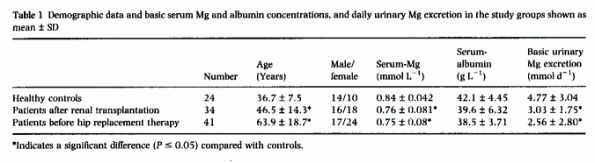
reported a sensation of warmth. Demographic data and values
obtained before the Mg-load are given in Table
1. Serum-Mg was significantly lower in patients but
their mean level was within the normal range in all groups. Basic
Mg-excretion and serum albumin concentration did not differ in
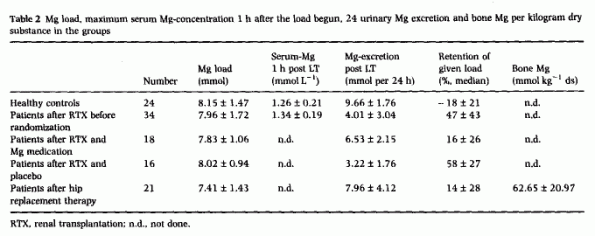
the groups. One hour after the infusion began, the maximum
serum-Mg concentration was not different between healthy controls
and patients (Table 2). After the load, 24 h
urine volume did not differ significantly in the patients (1878
± 769 mL) from the controls (1730 ± 610 mL). In
healthy subjects, the percentage of Mg retained was -18 ±
21, which is comparable to that obtained in a larger study: - 19
± 27.5 [4]. In patients 4 months after renal
transplantation, 47 ± 43% of the Mg load was retained,
which is the mean plus three standard deviations of the control
group. Four months later, the percentage retention decreased
towards normal values in patients who were Mg supplemented
(n = 16), but decreased in the placebo group (n
= 16, P = 0.001). Complete data were available for
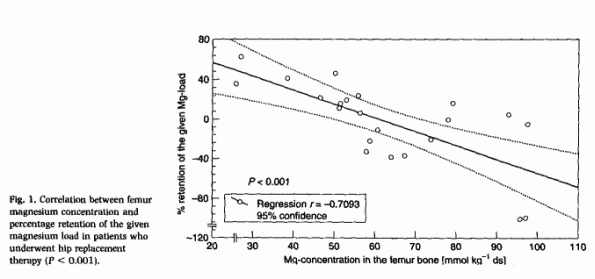
analysis from 21 patients after hip replacement; they retained 14
± 28% of the Mg load, and the Mg concentration in the
stored slice of the femur neck was 62.65 ± 20.97 mmol Mg
per kilogram dry substance. The correlation coefficient between
both was r = - 0.7093 [P < 0.001;
Fig. 1], whereas there was no significant
correlation between serum levels and bone Mg content (r
= 0.2217).
Discussion
No adverse reaction was encountered using the short-term
version of MLT: administration of 0.1 mmol Mg/kg as Mg aspartate
hydrochloride in 500 mL saline over 1 hour. Outpatients had no
problems related to the sampling and measuring of the urine
volume and the mailing of an aliquot. In healthy individuals, the
percentage retention of Mg was comparable with that obtained with
30 mmol (as MgSO4 in 500 mL saline) over 8 hours [4]. The
negative values of retention means that the amount of excreted
magnesium is greater than the Mg load. This results from the fact
that we did not take into account baseline Mg excretion which is
possible [22], but needs a second 24 h urine sampling opposing
the aim of this study to simplify the test. The broad range can
be the result of the fact that dietary Mg intake was not
standardized, in view of the difficulty in enforcing strict
nutritional restrictions in outpatients, and the fact that there
are thus resultant broad day-to-day variations in Mg excretion.
Peak serum Mg concentrations were lower in this study than in
that reported by Gullestadt et al. [4; 1.85 ±
0.54 mmol L-1 Mg]. This may be advantageous, since the higher the
maximum magnesium concentration, the greater the possibility of
overcoming maximum capacity for magnesium reabsorption in the
renal tubular system (Tm Mg) to give false negative results
because Tm Mg is close to the upper level of the normal serum
magnesium concentration [23]. Groups 2 and 3 were also
normomagnesemic regarding the mean value, but at the lower limit
of the Mg reference value in our laboratory (1.15-0.75 mmol L-1).
Patients after renal transplantation were outpatients in whom Mg
depletion might have developed due to renal Mg wasting [20]
related to a defective renal tubular system because of ischemia
and the nephrotoxicity of various drugs especially ciclosporine
[7, 10, 16]. The loading-test was applied to answer the question
of whether there is magnesium deficiency or not. Despite a normal
serum Mg level, we found a high percentage retention of the Mg
load which was greater than the mean plus 3 SD of the control
population indicating magnesium deficiency [7]. The validity of
the long-term Mg loading test version of Gullestad et
al. [4] to detect Mg deficiency was demonstrated by a
decrease in magnesium retention in a second loading test in the
same individuals some months later, after continuous oral
magnesium medication [18]. In our study, too, the percentage
retention of the Mg-load turned towards normal values after 4
months of Mg application. However, in patients to whom no Mg was
given there was even a higher retention rate of the given Mg
load, giving evidence for the assumption of magnesium deficiency.
Finally, we could show that the results obtained with the short
term MLT reflects, very well, bone Mg content, which is the most
relevant Mg store. Based on the above given data we conclude that
one can really measure Mg deficiency in outpatients using this
version of the MLT.
References
1 Wacker WEC, Parisi AF. Magnesium metabolism. N Engl J
Med 1968; 278: 658-63;712-7;772-6.
2 Elin RJ. Assessment of magnesium status. Clin Chem
1987; 33: 311-4.
3 Alfrey AC, Miller NL, Butkus D. Evaluation of body magnesium
stores. J Lab Clin Med 1974; 84:
153-62.
4 Gullestad L, Midtvedt K, Dolva LO, Norseth J. Kjeskshus J.
The magnesium loading test: reference values in healthy subjects.
Scand J Clin Lab Invest 1994; 54:
23-31.
5 Whang R, Ryder KW. Frequency of hypomagnesemia and
hypermagnesemia. Requested vs routine. J Am Med Assoc
1990; 263: 3063-4.
6 Dyckner T, Wester PO. The relation between extra- and
intracellular electrolytes in patients with hypokalemia and/ or
diuretic treatment. Acta Med Scand 1978;
204: 269-82.
7 Rob PM, Bley N, Dick K, Rohwer J. Sack K. Magnesium
deficiency after renal transplantation and Cyclosporine treatment
despite normal serum magnesium concentration detected by a
modified magnesium-loading-test. Transplant Proc 1995;
27: 3442-3.
8 Elin RJ. Magnesium metabolism in health and disease. Dis
a Month 1988: 34: 161-218.
9 Durlach J. New trends in international magnesium research.
Mag Res 1992; 5: 14-6.
10 Bley N. Dick K, Rob PM, Sack K. Follow-up measurement of
serum, lymphocyte and red blood cell magnesium concentrations
after renal transplantation and cyclosporin treatment with or
without magnesium supplementation. In: Smetana R., eds.
Advances in Magnesium Research London: John Libbey,
1997; 218-22.
11 Vormann J, Gunther T. Perras B. Rob PM. Magnesium
metabolism in erythrocytes of patients with chronic renal failure
and after renal transplantation. Eur J Clin Chem Clin
Biochem 1994: 32: 901-4.
12 Elin RJ. Assessment of magnesium status. In: Itokawa Y,
Durlach J, eds. Magnesium in Health and Disease. London:
John Libbey, 1989; 137-46.
13 Fitzgerald MG, Fourman P. An experimental study of
magnesium deficiency in man. Clin Sci 1956;
15: 635-47.
14 Thoren L. Magnesium deficiency in gastrointestinal fluid
loss. Acta Chir Scand 1963; 306
(Suppl.): 1-65.
15 Dyckner T. Wester PO. Magnesium deficiency - guidelines for
diagnosis and substitution therapy. Acta Med Scand 1982:
661 (Suppl.): 37-41.
16 Rasmussen HS, McNair P, Goransson L, Balslav S, Larsen OG,
Aurup P. Magnesium deficiency in patients with ischemic heart
disease with and without acute myocardial infarction uncovered by
an intravenous loading test. Arch Intern Med 1988;
148: 329-32.
17 Cohen L, Kitzes H. Characterisation of the magnesium status
of elderly people with congestive heart failure, hypertension,
and diabetes mellitus by the magnesium-load test. Magnes
Bull 1993; 15: 105-7.
18 Gullestad L, Dolva LO, Waage A, Falch D. Fagerthun H,
Kjekshus J. Magnesium deficiency diagnosed by an intravenous
loading test. Scand J Clin Lab Invest 1992;
52: 245-53.
19 Dick K, Bley N, Seyfert T, Dibbelt L. Rob PM. How to
diagnose magnesium deficiency in outpatients. Trace Elem
Electrolyte 1998; 15: 8-10.
20 Scoble JE. Freestone A, Varghese Z. Fernando ON, Sweny P,
Moorhead J. Cyclosporine-induced renal magnesium leak in renal
transplant patients. Nephrol Dial Transplant 1990:
5: 812-5.
21 Vormann J, Forster C, Zippel U, et al. Effects of magnesium
deficiency on magnesium and calcium content in bone and cartilage
in developing rats in correlation to chondrotoxicity. Calcif
Tissue Int 1997: 61: 230-8.
22 Danielson BG, Johansson G, Ljunghall S. Magnesium
metabolism in healthy subjects. Scand J Urol Nephrol
1979; 51 (Suppl.): 49-73.
23 Rude RK, Ryzen E. The Tm Mg and renal Mg threshold in
normal man and in certain pathophysiologic conditions.
Magnesium 1985; 5: 273-81.
Correspondence: Priv. Doz. Dr med. P.M. Rob, Ratzeburger Allee
160, D-23538 Lübeck, Germany (fax: 0049-451-5006334: email:
rob@medinf.mu-luebeck.de).
This page was first uploaded to The Magnesium Web Site on July
30, 2005
http://www.mgwater.com/



