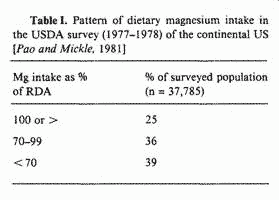Magnesium 5: 1-8 (1986)
Magnesium Content of the Food Supply in the Modern-Day
World1
J.R. Marier
Division of Biological Sciences, National Research Council,
Ottawa, Canada
Presented at the International Symposium on 'Magnesium and Its
Relationship to Cardiovascular, Renal, and Metabolic Disorders',
Los Angeles, Feb. 12 1985.
Key Words. Magnesium · Diet ·
Drinking water · Food refining · Vegetarians
· Hypertension · Stress · Cardiac ·
RDA
Abstract. A large-scale US survey has shown
that the dietary magnesium intake tends to be lower than
recommended. The suboptimal intake prevalent among US adults is
consistent with the pattern observed in other North American
and European surveys. Several factors are discussed, including
the waterborne magnesium factor, the loss of magnesium during
food refining and the magnesium content of vegetarian diets, as
well as various metabolic situations, e.g., hypertension,
pregnancy, osteoporosis, drug therapy, alcoholism, stress and
cardiac trauma. The benefits of magnesium supplementation among
those with sub-RDA intakes are illustrated.
In 1977-1978, the US Department of Agriculture (USDA)
conducted a 'Nationwide Food Consumption Survey' which showed
that the dietary intake of magnesium in the continental US tends
to be lower than recommended [38]. The pattern is illustrated in
table I. Note that more than 37,000 individuals were surveyed
(including all age groups from the newborn to the elderly), but
that only 25% of these persons had a dietary magnesium intake
that equalled or exceeded the Recommended Dietary Allowance (RDA)
proposed by the US National Academy of Sciences. In contrast, 75%
of the individuals did not meet the RDA criterion, and this group
was about equally divided between those whose magnesium intake
was from 70 to 99% of the RDA, and others with intakes that were
less than 70% of the RDA, In particular, it must be emphasized
that 39% of the subjects were in the latter category of lowest
magnesium intakes.
Another aspect of the USDA data reveals that the lowest intake
group consisted mostly of teenagers and adults [38]. On this
basis, it can be calculated that the dietary magnesium averaged
76% of the RDA among those aged 19 or more, and this translates
into the quantitative per diem intakes shown in table II. It can
be seen that the suboptimal intakes observed in the USDA survey
are compatible with those reported in other surveys conducted in
various regions of North America and Europe [34]. However, bear
in mind that the USDA data represent an average for the entire
continental US, but that much lower magnesium intakes have been
reported in some geographical regions such as the Newfoundland
area of Canada; and also among institutionalized elderly
populations. In such cases, the average magnesium intake can be
as low as 143-189 mg/day (i.e. only about 50% of the RDA), and
the daily shortfall in magnesium intake can be double the amount
derived from the USDA data. Here, it is important to emphasize
that - on an overall basis - the shortfall in magnesium intake
tends to range from 72 to 161 mg/day (see table II).

Some critics might claim that the magnesium status of
modern-day populations is more adequate than indicated by the RDA
criterion. But if indeed the magnesium status of modern-day
populations was adequate, it would be impossible to observe the
waterborne magnesium factor which provides an extra source of
daily magnesium intake and has been seen to reduce the
mortality/morbidity patterns on a global basis [35]. For cardiac
cases in the Ontario region of Canada, it has been calculated
that a daily magnesium supplement of 159 mg (in addition to the
normal dietary intake) would be required to prevent efflux of
myocardial magnesium [35]. This dosage is identical to the upper
limit of the shortfall range shown in table II, and suggests that
individuals with very low magnesium intakes may be at particular
risk from cardiac-related trauma.
The question of why the modern-day world's magnesium intake is
suboptimal has been addressed in previous reviews [1, 32, 33].
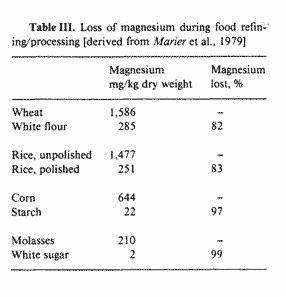
Basically, this situation has evolved because of an increased
dependency on overly refined and/or processed cereal-crop and
carbohydrate food staples in which the preponderance of the
magnesium has been removed (see table III). Given this
impoverished magnesium content of traditional dietary staples, it
is difficult to provide additional magnesium by recourse to
alternative modern-day food sources. Substances such as nuts,
chocolate, beans, shrimp and bran all contain elevated
concentrations of magnesium [44], but are not easily incorporated
into a daily dietary regimen. In comparison, meats and eggs are a
relatively poor source of magnesium. It is true that milk and
cheese can con tribute a sizeable proportion of the dietary
magnesium [2, 38]. However, these dairy, products provide about
10 times more calcium than magnesium (i.e. about 6 times more
calcium on a molar basis), and this factor might be important if
one wants to avoid calcium supplementation while replenishing a
magnesium deficiency [see ref. 8, 13]. Moreover, for those who
advocate a 'protective' effect of calcium in syndromes such as
hypertension and heart disease, it is well to remember the
epidemiological pattern in Finland where the per capita dietary
intake of calcium is among the highest in the world [i.e. 1,300
mg/day; see ref. 22], but where there is an exceptionally high
death rate from cardiovascular diseases [26].
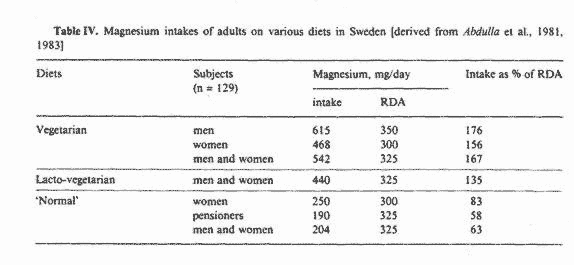
Recent surveys in Sweden [1, 2] have indicated that vegetarian
diets provide a more than adequate intake of magnesium, as
illustrated in table IV. Note that the magnesium intake of men
and women on vegetarian diets was considerably greater than the
RDA requirement; this was attributed to the high intake of
vegetables and whole-grain cereals by these individuals. In
comparison, table IV also shows that the magnesium supplied by
'normal' omnivorous diets was only 58-83% of the RDA. Although
these vegetarian diets tended to be particularly low in vitamin B
it is nevertheless of interest to note that the vegetarians had
lower levels of blood lipids (i.e., cholesterol, triglycerides
with low LDL/HDL ratios) than subjects on 'normal' diets. Also,
the vegetarians' blood pressure averaged 120/80, which is low for
their age group of 49-55 years.
Similar results have been reported from Australia [42a], where
the prevalence of mild hypertension was found to be 10% among
those ingesting an omnivorous diet, but only 1.5% among
vegetarians. The significant fall in blood pressure associated
with a vegetarian diet averaged 7 mm Hg systolic and 3 mm Hg
diastolic. Vegetarians also had lower serum cholesterol levels.
In comparison with the omnivorous regimen, the lacto-vegetarian
diets supplied 34% more magnesium [42a], and this is compatible
with the Swedish data presented in table IV.
The researchers in both Sweden and Australia wondered if the
antihypertensive effect of vegetarian diets might be due to a
higher intake of poly-unsaturated fatty acids, or fiber, or
magnesium or other minerals. There is, however, compelling
evidence in favor of a crucial role for magnesium.
In Hungary, a daily magnesium supplement of 80-160 mg resulted
in a decreased blood pressure in 74 industrial workers during a
6-week period; the fall in blood pressure averaged 15 mm Hg
systolic and 10 mm Hg diastolic [see ref. 29]. In Sweden,
clinical supplementation of magnesium (i.e. 360 mg/day,
equivalent to the RDA requirement) reduced both the systolic and
diastolic blood pressure by an average of 12/8 mm Hg during a
6-month period [see ref. 14]. A collaborative study involving US
and German researchers has provided experimental evidence
demonstrating that increasing the severity of magnesium
deficiency produces graded elevations of arterial blood pressure
[4]. A clinical study in the US has demonstrated that there is
significant depletion of intracellular ionic magnesium in
erythrocytes of humans with essential hypertension, with an
inverse linear relationship between intracellular Mg and
diastolic blood pressure [40]. In Finland, the use of a
magnesium-supplemented table salt has resulted in a fall in blood
pressure, averaging about 7 mm Hg systolic and 2 mm Hg diastolic
[see ref. 27]. This latter result is almost identical with the
effect of a lacto-vegetarian diet in Australia [42].
Mention has been made of the fact that institutionalized
elderly populations can have low magnesium intakes that account
for only about 50% of the RDA. This same situation applies to
pregnant women [cf. ref. 34] and also to postpartum women,
whether or not they are breast-feeding [36]. At this point, it is
pertinent to mention that a magnesium deficiency status has been
reported in postmenopausal women who have osteoporosis [10 see
also ref. 12].
Another 'low magnesium' subgroup of the population consists of
women who are engaged in athletics. Thus, the magnesium intake of
young adult female athletes in Indiana was only 68% of the RDA
[23] and less than 66% of the RDA among female collegiate
gymnasts in Salt Lake City [31] and among female students in
Fairbanks, Alaska [46]. The concern about low magnesium intakes
among people engaged in sports activities was the subject of a
recent discussion in France [37], and the basis for this concern
can be traced back to Selye's work on stress. Recent
biochemical studies with experimental animals in Canada [47] and
the US [5] have shown that magnesium deficiency decreases the
capacity of an animal to withstand prolonged exercise, but that
all activities involving endurance stress were enhanced by
magnesium supplementation. In view of the generally low magnesium
intakes prevalent in modern-day populations, along with the
current emphasis on 'jogging' and other forms of exercise, one
can only wonder about the long-term outcome.
Superimposed on this entire pattern is the fact that specific
clinical syndromes can induce magnesium deficiency [17, 48], and
that metabolic magnesium depletion can result from chronic use of
digitalis or diuretics and from alcohol abuse [11, 17, 41, 48].
An alert was published on this topic in 1970 by Becking and
Morrison [7] of Health and Welfare Canada:
'The occurrence of hypomagnesemia in humans, due in part to
prolonged use of diuretics, alcoholism, pregnancy etc., has been
shown to be more prevalent than earlier reports would have
indicated.'
In this context, it is necessary to point out an additional
modern-day problem; significant decreases in serum magnesium have
been reported among women using oral contraceptives [18, 43].
Clinically, slow-rate infusion of magnesium (thereby doubling
or tripling the serum magnesium concentration) has been used
successfully in the management of premature labor seizures of
pregnancy eclampsia [16, 39], and in the treatment of
cardiac-related trauma [15, 30, 33, 34]. Oral magnesium
supplementation has been used to inhibit formation of kidney
stones [24, 33] and to prevent postsurgical cardiac arrhythmias
[28].
In a recent publication [45], it was reported that patients
who have a magnesium intake below the US RDA requirement respond
with an improved clinical condition after magnesium
supplementation. Table V presents a list of human conditions that
have been improved by oral magnesium supplementation. Aside from
the situations already mentioned, this tabulation includes
metabolic conditions that seem to be particularly relevant to the
modern-day world. The effectiveness of magnesium supplementation
in all of these conditions cannot be attributed to mere
coincidence, but rather suggests that the low dietary magnesium
status prevalent in the modern-day world's adults increases their
susceptibility to a variety of ailments, many of which involve
impaired resistance to stress-induced tension.
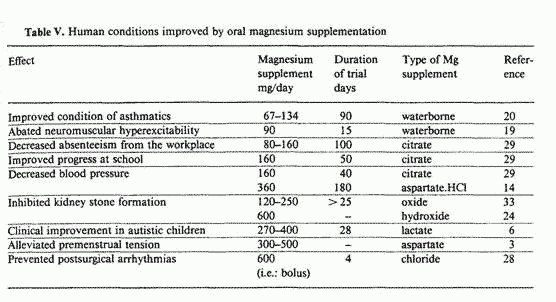
It is particularly interesting to note that, in 6 of the 9
metabolic conditions listed in table V, clinical improvement was
achieved with supplemental magnesium dosages in the range of
67-160 mg/day, i.e. almost identical with the 72-161 mg/day
'shortfall' in the magnesium intake of modern-day-world's adults,
as assessed by the US RDA criterion (see table II). This can be
taken as an indication that the US RDA is a reasonably accurate
index of metabolic magnesium requirements.
In contrast, a report issued by Health And Welfare Canada in
1983 [21] recommends daily magnesium intakes of only 240 and 190
mg by adult men and women, respectively. Both these values are
110 mg below the US RDA criteria. The only published basis for
the recent Canadian recommendation is a report from 1967 [25],
although close scrutiny of these data indicates a dietary
magnesium requirement of 280 mg/day for a mixed population of
adults [32], and graphic interpretation reveals that 360 mg/day
would be required to avoid the risk of a negative magnesium
balance in some individuals. At this point, it bears repeating
that recent studies [45] have shown that patients who have a
magnesium intake below the US RDA requirement respond with an
improved clinical condition after magnesium supplementation.
Therefore, in terms of everything that has been discussed in
this presentation, the modern-day world's dietary magnesium
status appears bleak, unless one happens to be a vegetarian or
has access to magnesium-rich drinking water (e.g., 30 to 90 mg/l
[see ref. 35]). As has recently been suggested [9], it is perhaps
time to return to the concept of 'subclinical magnesium
deficiency'. However, one of the main problems is that many
hospitals still do not perform routine magnesium analyses [see
ref. 42b, 48].
La teneur en magnesium de l'apport alimentaire
dans le monde d'aujourd'hui
Un examen aux USA sur une large échelle a montré
que l'absorption de Mg avec la ration tend à être
plus faible que celle qui est recommandée. L'absorption
suboptimale, qui prévaut chez les adultes des USA, est en
concordance avec les caractéristiques observées
dans d'autres examens aux USA et en Europe. Nous discutons de
l'importance de plusieurs facteurs, y compris le facteur du Mg
véhiculé par l'eau, la perte de Mg au cours du
raffinage des aliments et la teneur en Mg des regimes
végétariens, ainsi que de plusieurs situations
métaboliques, entre autres l'hypertension, la grossesse,
l'ostéoporose, la thérapeutique
médicamenteuse, l'alcoolisme, le stress et le traumatisme
cardiaque. Nous présentons les bienfaits d'un apport
supplémentaire de Mg chez les sujets avec un apport
suboptimal de la ration.
References
1 Abdulla, M.; Andersson, I.; Asp, N.C.; Berthelsen, K.;
Birkhed, D.; Dencker, I.; Johansson, C.G.; Jugerstad, M.; Kolar,
K.; Nair, B.M.; Ehle, P.N.; Norden, A.; Nassner, S.; Akesson, B.;
Ockerman, P.A.: Nutrient intake and health status of vegans -
chemical analyses of diets using the duplicate portion sampling
technique. Am. J. clin. Nutr. 34: 2464-2477 (1981).
2 Abdulla, M.; Jagerstad, M.; Kolar, K.; Norden, A.; Schutz,
A.; Svensson, S.: Essential and toxic inorganic elements in
prepared meals - 24-hour dietary sampling employing the duplicate
portion technique; in Braetter and Schramel, Trace element
analytical chemistry in medicine and biology, vol. 2, pp. 75-86
(de Gruyter, Berlin 1983).
3 Abraham, G.E.: Nutrition and the pre-menstrual tension
syndromes. J. appl. Nutr. 36: 103-124 (1984).
4 Altura, B.M.; Altura, B.T.; Gebrewold, A.; Ising, H.;
Gunther, T.: Magnesium deficiency and hypertension: correlation
between magnesium deficient diets and microcirculatory changes in
situ. Science 223: 1315-1317 (1984).
5 Avakian, E.V.; Brilla, L.; Colburn, S.M.; Horvath, E.:
Effects of dietary magnesium deficiency and chronic
exercise-training on adrenal catecholamine concentration. Fed.
Proc. 43: 801(1984).
6 Barthélémy, C.; Garreau, B.; Leddet, I.;
Sauvage, D.; Domenech, J.; Muh, J.P.; Lelord, G.: Effets
cliniques et biologiques de l'administration orale du magnesium
seul ou du magnesium associé a la vitamine B6 sur certains
troubles observes dans l'autisme infantile. Thérapie
35: 627-632 (1980).
7 Becking, G.C.; Morrison, A.B.: Role of dietary magnesium in
the metabolism of drugs by NADPH-dependent rat liver microsomal
enzymes. Biochem. Pharmac. 19: 2639-2644 (1970).
8 Caddell, J.L.: Studies in protein-calorie malnutrition. New
Engl. J. Med. 276: 533-540 (1967).
9 Chadda, K.D.: Personal communication. Heart Inst., Long
Island Jewish Med. Center (1985).
10 Cohen, L.; Kitzes, R.: Infrared spectroscopy and magnesium
content of bone mineral in osteoporotic women. Israel J. med.
Scis 17: 1123-1125 (1981).
11 Cohen, L.; Kitzes, R.: Magnesium deficiency and References
digitalis toxicity. J. Am. med. Ass. 251: 730
(1984).
12 Dalderup, L.M.: The role of magnesium in osteoporosis and
idiopathic hypercalcaemia. Voeding 21: 424-434
(1960).
13 Dooling, E.D.; Stern, L.: Hypomagnesemia with convulsions
in a newborn infant. Can. med. Ass. J. 97: 827-831
(1967).
14 Dyckner, T.; Wester, P.O.: Effect of magnesium on blood
pressure. Br. med. J. 286: 1847-1849 (1983).
15 Ebel, H.; Gunther, T.: Role of magnesium in cardiac
disease. J. clin. Chem. clin. Biochem. 21: 249- 265
(1983).
16 Elliott, J.P.: Magnesium sulfate as a tocolytic agent. Am.
J. Obstet. Gynec. 147: 277-284 (1983).
17 Rink, E.B.: Magnesium deficiency etiology and clinical
spectrum. Acta med. scand., suppl. 647, pp. 125-137 (1983).
18 Goldsmith, N.F.; Goldsmith, J.R.: Epidemiological aspects
of magnesium and calcium metabolism. Archs envir. Hlth
12: 607-6 19 (1966).
19 Gueux, E.; Poenaru, S.; Duchene-Marullus, P.:
Electromyographic changes associated with high level of magnesium
in drinking-water. Magnesium Bull. 2: 154-156
(1982).
20 Haddad, Z.E.: Les effets d'une eau de table riche en
magnesium sur l'asthme bronchiale. Allerg. Immunol. 14:
11-16 (1982).
21 Health and Welfare Canada: Recommended nutrient intake for
Canadians, Ottawa 1983, pp. 116-120.
22 Henrikson, P.A.: Periodontal disease and calcium
deficiency. Acta odont. scand. 26: suppl. 50, pp. 1-132
(1968).
23 Hickson, J.; Schrader, J.; Cunningham, L.: Female athletes'
energy and nutrient intakes. Fed. Proc. 42. 803 (1983).
24 Johansson, G.; Beckman, U.; Danielson, B.G.; Fellstrom, B.;
Ljunghall, S.; Wikstrom, B.: Prophylactic treatment with
magnesium hydroxide in renal stone disease; in 'Urolithiasis,
clinical and basic research', pp. 267-273 (1981).
25 Jones, J.E.; Manalo, R.; Rink, E.B.: Magnesium requirements
in adults. Am. J. clin. Nutr. 20: 632- 635 (1967).
26 Karppanen, H.; Pennanen, R.; Passinen, L.: Minerals,
coronary heart disease, and sudden coronary death. Adv. Cardiol.,
vol. 25, pp. 9-24 (Karger, Basel 1978).
27 Karppanen, H.; Tanskanen, A.; Tuomilehto, J.; Puska, P.;
Vuori, J.; Jantti, V.; Seppanen, M.L.: Safety and effects of
potassium- and magnesium- containing low-sodium salt mixtures. J.
cardiovasc. Pharmacol. 6: S236-S243 (1984).
28 Krasner, B.S.; Girdwood, R.; Smith, H.: The effect of
slow-releasing oral magnesium chloride on the QT interval of the
electrocardiogram during open-heart surgery. Can. Anaesth. Soc.
J. 28: 329-333 (1981).
29 Kuti, V.: Magnesium and civilization diseases. Vitalstoffe
15: 163-166 (1970).
30 Lehr, D.: Magnesium and cardiac necrosis. Magnesium Bull.
1a: 178-191 (1981).
31 Lewis, C.G.; Eisenman, P.A.: Annual fluctuations in dietary
intake and nutritional status of elite female collegiate
gymnasts. Fed. Proc. 43: 869 (1984).
32 Marier, J.R.: Cardioprotective contribution of hard waters
to magnesium intake. Rev. Can. Biol. 37: 115-125
(1978).
33 Marier, J.R.; Neri, L.C.; Anderson, T.W.: Water hardness,
human health, and the importance of magnesium. Nat. Res. Council
Canada, Report 18581, 1979.
34 Marier, J.R.: Quantitative factors regarding magnesium
status in the modern-day world. Magnesium 1: 3-15
(1982).
35 Marier, J.R.; Neri, L.C.: Quantifying the role of magnesium
in the interrelationship between human mortality/morbidity and
water hardness. Magnesium 4: 53-59 (1985).
36 Moser, P.B.; Issa, C.F.; Reynolds, R.D.: Dietary magnesium
intake and the concentration of magnesium in plasma and
erythrocytes of postpartum women. J. Am. Coil. Nutr. 4:
387-396 (1983).
37 Niquet, G.; Milbled, G.; Fuerrin, F.; Choquel, D.; Hequet,
B.; Lipka, E.: Magnesium et sport. Larc. Medical 4:
165-1 72 (1984).
38 Pao, E.M.; Mickle, S.J.: Problem nutrients in the United
States. Fd Technol. 35: 5 8-69 (1981).
39 Pritchard, J.A.; Cunningham, G.; Pritchard, S.A.: The
Parkland Memorial Hospital protocol for treatment of eclampsia.
Am. J. Obstet. Gynec. 148: 95 1-963 (1984).
40 Resnick, L.M.; Gupta, R.K.; Laragh, J.H.: Intracellular
free magnesium in erythrocytes of essential hypertension.
Relation to blood pressure and serum divalent cations. Proc.
natn. Acad. Sci. USA 81: 6511-6515 (1984).
41 Reyes, A.J.; Leary, W.P.: Cardiovascular toxicity of
diuretics related to magnesium depletion. Human Toxicol.
3: 351-371 (1984).
42a Rouse, I.L.; Armstrong, B.K.; Beilin, L.J.; Vandongen, R.:
Vegetarian diet, blood pressure, and cardiovascular risk. Austr.
N. Z. J. Med. 14: 439- 443 (1984).
42b Ryzen, E.; Wagers, P.W.; Singer, F.R.; Rude, R.K.:
Magnesium deficiency in a medical ICU population. Crit. Care Med.
13: 19-21 (1985).
43 Saleh, F.M.; Elnein, A.E.; Souka, A.R.; Nasr, F.I.;
Khattab, A.A.: Study of serum levels of magnesium, calcium, zinc,
and iron in users of oral contraceptives. J. Kuwait med. Ass.
18: 23-27 (1984).
44 Seelig, M.S.: The requirements of magnesium by the normal
adult. Am. J. clin. Nutr. 14: 342-390 (1964).
45 Sheehan, J.P.; Sisam, D.; Schumacher, O.P.: Clinically
significant magnesium deficiency of dietary origin. J. Am. Coll.
Nutr. 3: 245 (1984).
46 Tallas, P.G.: Dietary intake of female college students in
Fairbanks, Alaska. Proc. Alaska Sci. Conf. 32:
14(1981).
47 Turcotte, R.A.; Gilchrist, J.; Quinney, H.A.; Belcastro,
A.N.: Cardiac myofibril ATPase activity of endurance-trained rats
and Mg regulation. Med. Sci. Sports Exercise 15: 179
(1983).
48 Whang, R.: Magnesium deficiency: causes and clinical
implications. Drugs 28: suppl. 1, pp. 143-150
(1984).
Received: March 12, 1985
Accepted: May 15, 1985
J.R. Marier,
Environmental Secretariat,
Division of Biological Sciences,
National Research Council,
Ottawa, Ontario, K1A 0R6 (Canada)
This page was first uploaded to The Magnesium Web Site on
August 31, 2002
http://www.mgwater.com/