Volume 3a, Modern Aging Research
Intervention In the Aging Process, Part A: Quantitation,
Epidemiology, and Clinical Research
Editors: William Regelson, F. Marott Sinex
1983, Alan R. Liss, Inc, New York
pages 279-305
Possible Role of Magnesium in Disorders of the Aged
Mildred S. Seelig, M.D., M.P.H., F.A.C.N.
GOLDWATER MEMORIAL HOSPITAL, MEDICAL DEPARTMENT
NEW YORK UNIVERSITY MEDICAL CENTER
ROOSEVELT ISLAND, NEW YORK, NEW YORK 10044
INTRODUCTION
Magnesium (Mg) is important in many reactions, and in
prevention and treatment of functional and structural disorders
of many tissues and systems. There are numerous recent
publications on its effects on enzymes, in subcellular and
cellular preparations, and in plants and animals, including man.
However, relatively little has been done on Mg in aging. It is
necessary to draw largely from studies that show changes in Mg
deficiency that resemble those of old age, and relate Mg
requirements to deficiencies of other nutrients particularly
those with which Mg interacts. It has been postulated that Mg
deficiency early in life gives rise to chronic abnormalities that
persist throughout life, increasing morbidity and mortality and
shortening life (Seelig, 1977; 1977/1982; 1978; 1980). Little
attention has been paid to special Mg needs of old people, to
whether Mg inadequacy might contribute to the aging process, or
to whether Mg supplementation might have any beneficial effects
in the aged.
MAGNESIUM REQUIREMENTS OF THE AGED
The general assumption that most Western diets are adequate in
Mg has been questioned since analysis of metabolic balance
studies disclosed that at intakes below 5 and 6 mg/kg/day in
young women and men respectively, maintenance of Mg equilibrium
is not consistent (Seelig, 1964). Analysis of numerous typical
sample meals of Americans of all ages has shown that the Mg
intakes are usually below the Recommended Dietary Allowance (RDA)
(U.S. Dept. Agr. 1980). The RDI for Mg has been estimated at
300-350 mg/day for young women and young men, (Food and Nutr.
Board, 1980), providing about 4.5-5 mg/kg/day. The RDAs may not
be optimal for everyday living. especially for the elderly, since
they are derived from balance studies with young healthy adults
under controlled stable conditions — usually protected from
the vicissitudes of life (Seelig, 1981). Studies to assess the
influence of age: (psycho-social, physical, chronic disease and
therapy) on the Mg needs have not been done. It is probable that
Mg requirements are elevated in the elderly, in view of the many
factors in old age that increase nutritional needs and interfere
with utilization (Figure 1).

Magnesium Intake, Absorption and Excretion
The Mg intake of old people tends to be low (U.S. Dept. Agr.,
1980; Vir & Love, 1979), and their intestinal absorption of
Mg declines gradually with increasing age (Mountokalakis et al,
1976); Johansson, 1979). Lower urinary Mg excretion has been
reported by old than by young men. (Simpson et al, 1978). Young
women excreted less Mg than did post menopausal women, a
difference that was more marked in those taking oral
contraceptives (Table 1, Goulding & McChesney, 1977).
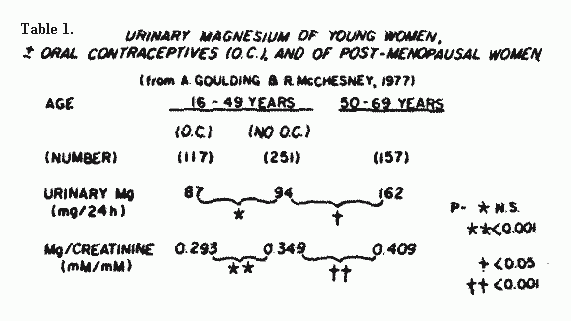
Serum and Tissue Levels of Magnesium
Serum Mg levels have been reported as quite constant in
healthy adults, regardless of age (Keating et al, 1969), and as
lower in old than young adults (Henrotte et al, 1976/1980). In a
study of circadian changes in serum Mg, young man exhibited lower
peak (morning) levels than did old men (Touitou et al, 1978). All
subjects had their lowest serum Mg levels during sleep hours at
night. The greatest circadian amplitude was in old men. In an
on-going study of chronically hospitalized patients, most of whom
are old, low Mg levels and high Mg retention after parenteral
loading are being encountered (Seelig & Berger,
unpublished).
Increased estrogen levels, or administration of estrogen,
caused both reduced serum levels and urinary output of Mg
(Goldsmith et al, 1970; Goldsmith & Johnston, 1976/1981).
These effects are attributed to estrogen-induced Mg shift to
tissues. The bone loss of post-menopausal women has been
correlated with the loss of bone matrix Mg, as well as of calcium
(Ca); the higher incidence of thrombotic events in young .women
and the increased incidence of cardiovascular disease in old
women might be due to the shift of Mg from blood plasma in young
and the loss of cardiac Mg in old woman (Goldsmith &
Goldsmith, 1966; Goldsmith & Johnston, 1976/1980; Seelig,
1980).
Mg is predominantly an intracellular cation, and serum levels
are an unreliable index of its status in the body (Walser, 1967;
Seelig, 1980; Wacker, 1980). Cardiac integrity being particularly
vulnerable to Mg loss (Seelig, 1972; Seelig & Heggtveit,
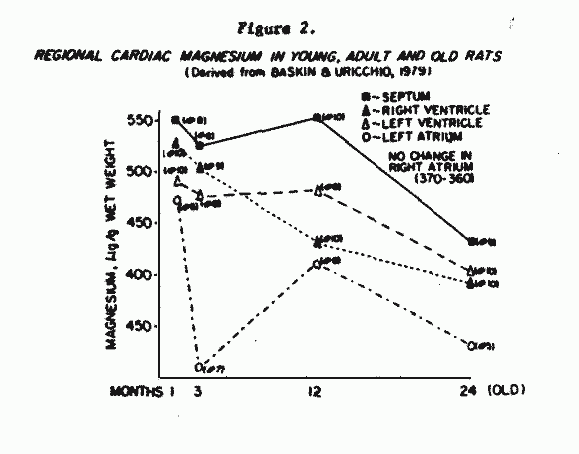
1974), the drop in myocardial Mg seen in aging rats may be
germane to the high cardiac disease rates in the aged. There were
striking reductions in myocardial Mg levels of old versus young
female rats. The septum and ventricles of male rats lost the most
Mg with increasing age (Figure 2). The Mg reduction was
accompanied by lesser falls in Ca and potassium (K), but not in
phosphate (P) levels. The cation changes ware not related to
dilutional factors, as the oldest rat. had the lowest tissue
water levels. There were significantly lower Mg levels in aorta
and liver of old rats than in young, bat little change in
skeletal muscle or renal Mg in a study in which renal Ca fell
with age (Mori & Duruisseau, 1960). In another study in which
renal Ca rose substantially with age, renal Mg fell (Baskin et
al, 1981). Magnesium retention has been shown to decrease in
senescent mice (Draper, 1964).
NUTRIENT/MAGNESIUM INTERRELATIONS IN THE AGED
The intakes of most nutrients by the elderly decline
strikingly, especially in the seventh decade; the greatest
decreases in the macronutrients are in fat and protein, with only
small carbohydrate decreases (Exton-Smith, 1970; Crapo, 1982).
Decreased physical activity is correlated with reduced energy
requirements of old age (McGandy et al, 1978). Protein needs,
however, rise with increasing age (Munro & Young, 1978; Uauy
et al, 1978), which makes the carbohydrate intake
disproportionately high. Each of these nutrients affect the Mg
requirements as do several of the vitamins — low levels and
poor utilization of which have been found in the elderly (Oldham,
1962, Baker et al, 1979; 1980).
Fat, Sugar and Protein: Interrelations with Magnesium
Fat. Interference with Mg
absorption by high intakes of saturated fat was demonstrated long
ago (Sawyer et al, 1918); high intestinal fat has contributed to
hypomagnesemia and resultant arrhythmia in patients with
steatorrhea (Chadda et al, 1973). Fats of different chain length
and degrees of saturation affect Mg absorption differently
(Rayssiguier, 1981). Experimental studies of the effects of Mg on
plasma lipids have yielded conflicting results, depending on the
dietary mix and the species used. Early rat studies showed that
Mg supplements exert a greater protective effect against fat
deposition (in heart and arteries) than against hyperlipidemia
and lowered lipoproteins more than liproproteins (Vitale et al.,
1966; Hellerstein et al., 1960). A more recent study has shown
that rats fed a Mg deficient diet that was rich in fat developed
hypertriglyceridemia and significantly lower levels of high
density lipid cholesterol (HDL-C) (Figure 3, Geux and
Rayssiguier, 1981). The cholesterol-rich diet did not alter serum
Mg levels appreciably. Pigs fed a diet low in Mg developed
elevated serum triglycerides (Nuoranne et a]., 198O) Young women,
who lost an average of 63 mg/day of Mg while on a diet providing
4.2-5.4 mg/kg/day (the RDA), showed rising blood lipids even
though the dietary fat was low: 1 g/day (Irwin and Feeley, 1967).
The authors recommended increasing the RDA for Mg.
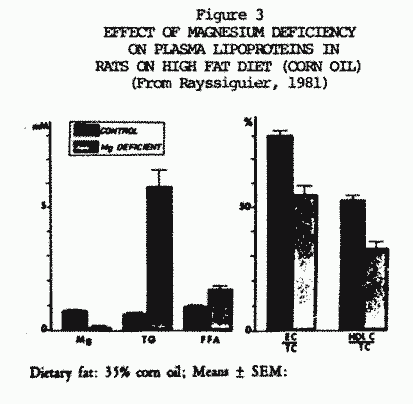
Even though serum Mg levels do not correlate reliably with
lipid levels in patients with atherosclerosis + hyperlipidemia,
Mg treatment of patients with myocardial infarction has been
reported to lower the LDL-C, to raise the HDL-C, and to produce
clinical improvement (Seelig, 1980: chapter 5; Rayssiguier,
1981).
Sugar. High sugar intakes directly
increase urinary excretion of Mg (Lindeman et al, 1967; Lennon et
al., 1974). Perhaps the Mg loss caused by sugar contributed to
the hypertriglyceridemia of Mg deficient rats fed a high sucrose
diet that was not rich in fat (Figure 4: Rayssiguier, 1981).
Diets disproportionately high in carbohydrate increase thiamim
needs, which can increase Mg requirements (Infra
vide).
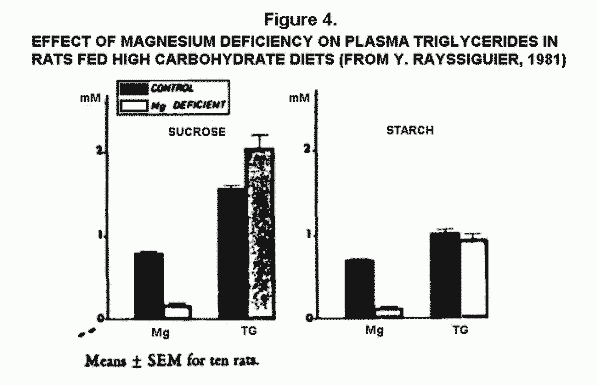
Protein. Unduly low protein intakes
have been shown to cause negative Mg balances in adolescent boys
(Schwartz et a]., 1973), and in young adults (Hunt and Schofield,
1969; McCance et al, 1942); increased protein intake improved the
retention of Mg. However, protein loading has increased Mg loss
(Lindeman et al., 1976/1980). Of importance for the elderly whose
financial status often precludes increasing protein intakes
substantially, is the finding that supplementing low to marginal
protein diets with Mg (increasing the Mg to optimal or above)
improved the retention of nitrogen of young people (McCance et
al, 1942; Schwartz et al., 1973).
Vitamins with Interrelationships with Magnesium.
Thiamin. Mg deficiency interferes
with responsiveness to thiamin in rats (Itokawa et al., 1974;
Zieve et al, 1968). Correction of the Mg deficit has restored
thiamin responsiveness in alcoholics with encephalopathy
(Stendig-Lindberg, 1972). The Mg-dependence of thiamin
utilization is a consequence of the role of Mg as a cofactor in
enzymes requiring thiamin (Vallee, 1960). Additionally, evidence
has been presented that Mg plays a role in binding thiamin with
tissue protein (Itokawa et al, 1974). Thiamin deficiency also
inhibits Mg utilization. It may be clinically important that Mg
deficient rats with normal thiamin intake had lower plasma and
tissue Mg levels than did those with double deficiency. (Figure
5, Itokawa, 1972).
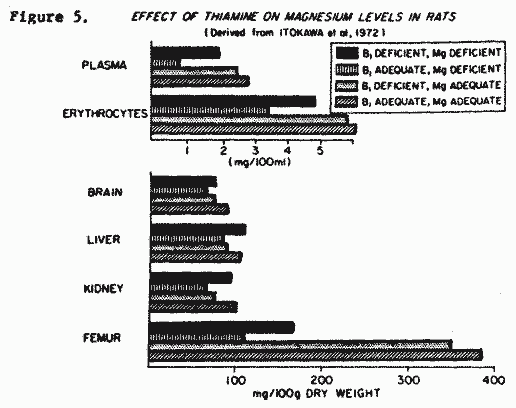
It seems plausible that efforts to repair the B1.
deficiency in aged patients with or without alcoholism carry the
risk, not only of a poor response to thiamin, but of intensifying
Mg deficiency. The studies that show more vulnerability to
thiamin deficiency in older than young subjects, and a need for
higher intakes to correct the inadequacy (Oldham, 1962) did not
provide data on the status of Mg. Studies of the effect of Mg
supplements on the response of patients with vitamin
B1. deficiency are needed.
Pyridoxine. In early Mg deficiency
studies, it was found that a pyridoxine deficiency (sometimes
with riboflavin deficiency) resulted in more rapid induction of
the acute Mg deficiency syndrome (Greenberg, 1939). Experimental
B6 deficiency causes loss of tissue Mg (Aikawa 1960),
and has been associated with transitory hypermagnesemia (Durlach,
1969), perhaps with egress of Mg from tissues, and hypomagnesemia
(Rigo et al, 1967), when tissue Mg is depleted. Several of the
enzymes that require pyridoxal phosphate also require Mg as a
cofactor (Vallee, 1960). The similarity of syndromes of
experimental B6 and Mg deficiencies, and in the
clinical disorders resulting from their deficiencies (Seelig,
1981) are thus not surprising. Included. among disturbances in
which both Mg and B6 deficiencies might play a role
that are common in the aged are chronic anemia and calcium
urolithiasis. B6 dependent anemia (Frimpter et al,
1969) might also be dependent on Mg, as Mg deficiency has been
shown to cause damage to erythrocyte membranes (Elin, 1973,
1976/1980). B6 and Mg have been useful alone and in
combination with calcium urolithiasis (Gershoff and Prien, 1967;
Johansson et al, 1982). Requiring further study is the
possibility that Mg might prove useful in B6-dependent
disorders in which Mg-dependent enzymes are involved. Among
explanations of B6 deficiency in the aged, and the
occasional failure to return to normal after trytophan-loading,
is defective phosphorylation of the vitamin by pyridoxal
phosphokinase to its active form (Hamfelt, 1964). This is one of
the enzymes that requires Mg. Correction of pyridoxine deficiency
should thus entail correction of Mg deficiency (Table
2).
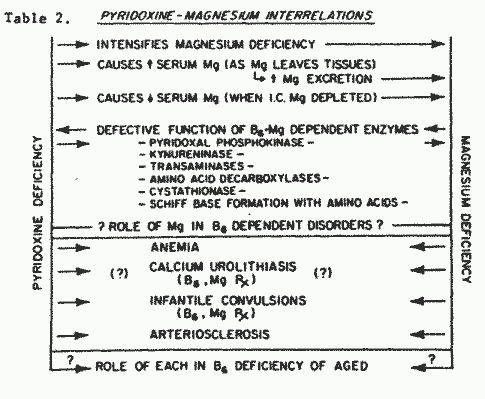
Interrelationships of zinc (Zn) with B6 and Mg are
also important. B deficiency causes loss of tissue Zn (Hsu,
1965), as well as Mg. Both Mg and Zn are needed for nucleic acid
synthesis and for the activity of many enzymes (Parisi and
Vallee, 1969). Zn is necessary for energy-linked Mg accumulation
by heart mitochondria (Brierley et al, 1967).
Vitamin E. Free radical damage to
membranes and to immune surveillance is implicated in the aging
process (Harman et al, 1977); both vitamin E as a free radical
scavenger, and Mg (Elin, 1976/1981) are important in maintaining
membrane stability. Interrelationships between the two are
indicated by the lowered tissue Mg levels in vitamin E deficient
animals (Blaxter and Wood, 1952) and manifestations of Mg
deficiency in vitamin E deficiency in rats (Schwartz, 1962). It
would be interesting to ascertain whether Mg administration can
protect against the free radical induced membrane damage
associated with lipid peroxides, and whether the postulated
slowing of the aging process by anti-oxidants (Tappel, 1968)
might be potentiated by Mg.
Vitamin D and Calcium. Experimental
Mg deficiency interferes with the utilization of vitamin D
(Lifshitz et al, 1967a), and vitamin D deficiency results in
decreased absorption of Mg and low serum Mg (Miller et al, 1964).
Clinical rickets has been associated with hypomagnesemia (Breton
et al, 1961). Correction of Mg deficiency has corrected vitamin D
refractoriness in children (Rosler and Rabinowitz, 1973; Reddy
and Sivakumar, 1974) and adults (Medalle et al, 1976). On the
other hand, excess vitamin D has intensified Mg deficiency in
animals (Lifshitz et al, l976b) and in clinical primary
hypomagnesemia (Paunier et al, 1968). Vitamin D hyperreactivity
causes hypercalcemia (Seelig, 1969), and high dietary Ca/Mg has
been implicated in cardiovascular disease (Karppanen et al,
1978). In the geriatric population, vitamin D deficiency and
hypocalcemia is more likely. Mg deficiency can contribute to both
by decreasing target organ responsiveness (Wallach,
1976/1981).
Fiber and Phytates Americans have
been advised to increase their intake of fiber because the
incidence of several chronic diseases is lower among population
groups on a high fiber diet than among those eating refined diets
(U.S. Senate Comm., 1978). Not generally realized is the
interference by phytates with the absorption of Mg (Seelig,
1981). Studies with natural fiber-rich foods, or with artificial
bulk substances added to the diet, have shown production or
increase of negative balance (Reinhold et al, 1976; Slavin and
Marlett, 1980). Elderly people commonly use phytate or other bulk
preparations to relieve their constipation. Their use, and the
abuse of purgatives other than Mg salts, may well interfere with
Mg utilization.
RELATIONSHIPS OF SOME DISTURBANCES IN AGING TO MAGNESIUM
Among the changes prevalent in the old are some that resemble
abnormalities that are caused by Mg deficiency, alone or in.
combination with other modalities. Diseases to which the elderly
are vulnerable, and some of the drugs used in therapy, contribute
to Mg loss. Although the evidence is insufficient to conclude
that increasing Mg intake throughout life might delay changes in
senescence, it is worth investigating whether prophylactic and
therapeutic use of Mg might be beneficial.
Cardiovascular Diseases, Cardiotonics, and Diuretics
Cardiovascular disorders are the major causes of morbidity and
mortality in the population over 55. There is considerable
evidence that long-term Mg inadequacy, of degrees not reflected
by serum Mg levels considered subnormal, can contribute to
functional and structural cardiovascular disease (Seelig, 1978,
1980; Seelig and Heggtveit, 1974; Seelig and Haddy, 1976/1981).
Mg, rather than Ca, has been clearly demonstrated to be the
critical protective water-factor in epidemiologic studies of the
different cardiac mortality rates in hard and soft water areas
(Anderson et al, 1975; Neri and Marier, 1977/1982). Magnesium
deficiency or loss seems central to cardiovasomyopathy (Seelig,
1980: pages 135-264).
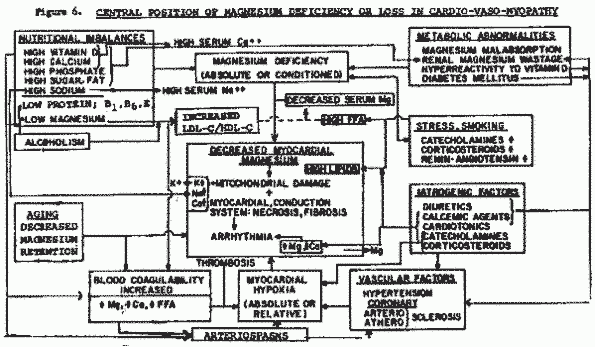
Coronary and peripheral vasospastic diseases have been
attributed to high dietary (Karppanen et al, 1978) and blood and
tissue Ca/Mg ratios (Altura et al, 1981; Altura, 1982). Numerous
in vitro studies have shown- the importance of Mg in regulating
contractility of arterial smooth muscle, including coronary and
cerebral arteries (Altura and Altura, 1980; 1981; Altura, 1982).
Hormone and neurotransmitter-induced vasoconstriction, that is
mediated by increased Ca, is inhibited by increased Mg. Lowering
Mg concentration allows for more Ca uptake by blood vessels;
raising Mg levels to above the usual serum concentration
decreases the Ca influx. Mg, thus, is a natural “calcium
blocker". It potentiates pharmacologic Ca-blocking effects on
arteries (Altura, 1982; Turlapaty et al, 1981) and has been found
to have greater anti-spasmotic activity than Verapamil in canine
coronaries (Altura, p.c.).
Those with congestive heart failure and arrhythmia can lose Mg
as a result of hypoxia — which causes Mg egress from
tissues, including the myocardium (Hochrein et al, 1967; Seelig,
1972; 1980, p. 193). This has been shown in hearts from animals
with occluded coronaries (Cummings, 1960; Jennings and Shen,
1972). Since cardiotonics stimulate Ca-inflow and Mg-outflow from
the heart, and inhibit Mg-dependent mitochondrial enzymes
(Seelig, 1972), it is not surprising that Mg deficiency and Ca
treatment intensify digitalis toxicity, whereas Mg treatment
counteracts it (review: Seelig, 1980, pp 255-259). The long-term
use of diuretics in cardiac or hypertensive patients, and in
those being treated for calcific urolithiasis, is the major
drug-induced cause of Mg loss (Wacker, 1980). Potassium loss is
always sought and treated in diuretic-treated patients; Mg loss
is less often considered. Such patients are prone to K-refractory
hypokalemia, sometimes with ectopic or premature ventricular
contractions, that are associated with decreased muscle K and Mg
levels, and that respond better when Mg is repleted than when K
alone is given. (Dyckner, 1980; Dyckner and Wester, 1981).
Transient ischemic attacks, that increase in prevalence with
increasing age, are associated with increased platelet
aggregation and thromboembolic events. There is in vitro evidence
that high concentrations of Mg inhibit platelet aggregation and
release (reviews: Elin, 1976/1981, Durlach, 1976/1981), and in
vivo evidence that Mg administration before temporary arterial
occlusion prevents platelet deposition on the injured endothelium
(Adams and Mitchell, 1979).
The protective effect of Mg was demonstrated in another study,
in which arterial thickening, due to fibrosis and smooth muscle
proliferation, was more marked in vessels of Mg deficient rats
than in controls (Rayssiguier, 1981).
Among the cardiovasopathic models that are protected against
by Mg, are the modified high fat diets that are thrombogenic
(Szelenyi et al, 1967; Savoie, l972a; Savoie and DeLorme,
1976/1981). In these studies, not only were lipid blood levels
increased, but Mg levels were decreased. Mg has been effective in
reducing the hypercoagulability of rats and dogs on thrombogenic
diets (Szelenyi et al, 1967; Savoie, l972b).
The increased incidence of thromboembolic events in women
taking estrogen-containing oral contraceptives has been biased on
the estrogen-lowering of plasma Mg (Goldsmith and Goldsmith,
1976/1981; Goldsmith and Johnston, 1976/1981). Patients with
latent tetany of marginal Mg deficiency have exhibited
phlebothrombosis (Durlach, 1967; Seelig et al, 1976/1981). The
data slowing Mg reduction of arteriospasm and of platelet
aggregation seem directly applicable to transient ischemic
cerebral and cardiac attacks; the data on Mg-protection against
arterial and cardiac damage seem relevant to the arteriosclerosis
and ischemic heart disease.
Diabetes Mellitus; Decreased Glucose Tolerance
Diabetes mellitus, which contributes to hyperlipidemia and
cardiovascular disease, and has been termed a model for aging
(Eckel and Hoefeldt, 1982), has long been known to be associated
with Mg loss (Martin et al, 1952; Jackson and Maier, 1968). A
decline in glucose tolerance is characteristic of aging. It has
been reported in Mg deficient rats (Rayssiguier, 1981). Insulin
refractoriness has improved with Mg therapy (Mules and McMullen,
1982; Seelig, unpublished data). Diabetic retinopathy has been
correlated with hypomagnesemia (McNair et al, 1978), and with
increased platelet aggregation (Heath et al, 1971). Since Mg
inhibits platelet aggregation, its administration to diabetics is
worth trying.
Collagen, Fibrosis and Aging
Collagen becomes more abundant, as well as more rigid, with
increasing age (Hall, 1969). Among the nutrients that influence
the metabolism of collagen are vitamins B6 and E,
which have interrelationships with Mg (supra vide). Mg deficiency
increases the cardiac fibrosis that is caused by noise
stress-induced catecholamine release (Gunther, 1981; Ising et al,
1981). The arterial fibrosis and the delayed uterine involution
and fibrosis of Mg deficiency, has been attributed to the slowing
of collagen turnover (Larvor, 1981; Rayssiguier, 1981).
Stress, Magnesium Loss and Cardiovascular Disease
Stress factors particularly likely to be encountered by the
aged include chronic anxiety and worry, and the acute stress of
bereavement. Regardless of the cause, stress increases
catecholamine and corticoid release, which in turn cause Mg loss.
Catecholamines also increase myocardial Ca uptake (Nayler, 1967).
Since low Mg/Ca ratios increase catecholamine secretion (Baker
and Rink, 1975), a vicious cycle is thus established when Mg
deficiency preexists. Well accepted is the contributory role of
stress to cardiovascular disease, including sudden unexpected
cardiac death. Less well known is the role of Mg loss in the
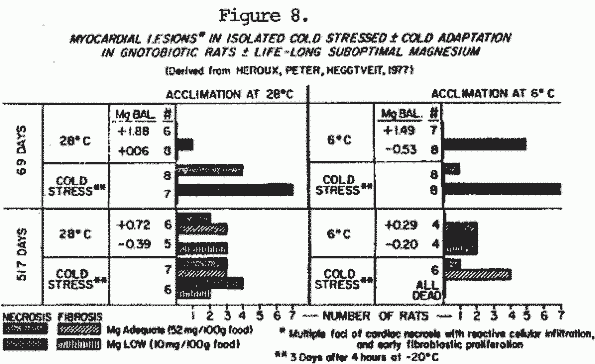
damage caused by stress (Figure 7). Long-term suboptimal Mg
intake, to which adaptation had taken place, so that signs of
deficiency that were present early no longer existed, resulted in
decreased tolerance of stress and shortened life expectancy
(Heroux et al, 1973).
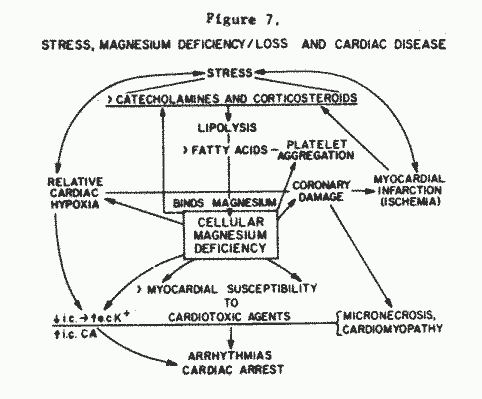
IMMUNOLOGY, ONCOLOGY AND INFECTION
With advancing age there are abnormalities in immune
regulation, most of which involve altered T-cell function, such
as lowered resistance to intracellular microbes, increased levels
of autoantibodies, and reduced immunosurveillance; i.e., against
neoplasm (Makinodan and Yunis, 1980). It is thus provocative that
Mg deficiency has been implicated in T-cell abnormalities and in
impaired protein synthesis (reviews: Seelig, 1979; 1980/1983).
Young rats with acute Mg deficiency developed lymphoid and
splenic hypertrophy (Hungerford and Karson, 1960), despite
significant reduction of protein synthesis by spleen and thymus,
little effect on RNA synthesis, but markedly increased splenic
and thymic lymphocyte DNA synthesis (Zieve et al, 1977). The
lowered lymphatic protein synthesis was correlated with impaired
immune response of Mg deficient rats; the increase in DNA
synthesis was considered representative of an early
1ymphoproliferative process leading to neoplasia, as has been
reported by others (Jasmin, 1963; Hass et al, 1976/1981). It is
interesting that the neoplasms were seen only in rats deficient
in Mg from early life, not in those made deficient when mature.
The lymphoma-producing diets were very high in Ca, with Ca/Mg
ratios of 140/1; diets that resulted in thymic hyperplasis, but
not thymoma provided a ratio of 10/1 (Alcock et al, 1973). Very
high Ca levels stimulate DNA synthesis and mitosis of cultured
human lymphocytes (review: Seelig, 1979).
Mg deficiency suppresses levels of most immunoglobulins in
rats and mice: IgG and IgA transiently, and antisheep red cell
hemolysin substantially (Larvor, 76/81; Alcock and Shils, 1974;
Elin, 1975; McCoy and Kenney, 1975). In contrast, IgE levels rose
3-4 fold (Prouvost-Danon et al, 1975). Fewer antibody-forming
cells and markedly less rosette formation by lymphocytes of Mg
deficient mice suggest the dependence on Mg by helper T-cells and
the impairment of T and B cell cooperation in Mg deficiency
(Guenounou et al, 1978).
Mg’ s interrelationship with other nutrients that affect
immunocompetence and immunosurveillance (reviews: Seelig, 979;
1980/1983), such as interactions among Mg, Zn and B6,
might influence reactions of the aged. Interrelationships among
agents that protect membrane stability, such as Mg, vitamin E,
Zn, and selenium might protect against oncogenesis. It must be
cautioned that Mg supplementation of patients with cancer is not
recommended, in view of the evidence that Mg depletion has
inhibited the growth of experimental and clinical advanced
neoplasm Young and Parson, 1977).
Infectious diseases cause about a third of all death in the
aged, particularly those involving the urinary tract,
endocardium, lungs and skin (Mostow, 1982). Impaired host
defenses make the facultative pathogens a particular risk. Such
microbes often require treatment, with antibiotics such as the
tetracyclines and, aminoglycosides — both of which classes
of drugs cause Mg loss. The tetracyclines chelate Mg (Shils,
1962); the aminoglycosides increase renal excretion of Mg as a
result of the tubular damage (Keating et al, 1977).
NEED FOR STUDY
To what extent intakes of Mg, insufficient to meet the special
needs of the aged, can increase susceptibility to disorders with
manifestations comparable to those produced by Mg deficiency
requires study. Complicating such studies will be the many
factors that affect Mg requirements, and that are a particular
problem in the elderly. In considering intervention studies that
might lead to improved quality and possibly length of life,
methods to evaluate long-term Mg supplementation should be
developed. Serum Mg determinations are unlikely to yield
revealing data, usually only profound deficiencies causing
hypomagnesemia. Percentage retention of parenterally administered
Mg is more rewarding, but it is not appropriate for large-scale
screening tests. Simplified means to measure cellular Mg: i.e. in
white blood cells are under study (Ross et al, 1976/1981; 1982;
Elin and Johnson, 1981; Ryan et al, 1981).
Other parameters, that should provide useful data on
Mg-induced changes, include changes in HDL-C/LDL-C ratios.
Electrocardiographic monitoring of patients on diuretics for
correction of occult EGG changes, in association with
Mg-correction of refractory hypokalemia, and in those whose
arrhythmias do not respond to K repletion, should be employed in
high risk patients.
Important clues to the poor adaptation to stress of the aged,
might derive from extension of the important study that showed
that young rats with Mg deficiency, adapted to sustained low Mg
intake and ceased showing signs of deficiency (Heroux et al,
1973). The tolerance of stress by surviving old Mg deficient rats
was significantly less than was that of control rats — in
terms of cardiac necrosis and survival. (Figure 8). Also, their
lives were shorter, even without stress.
It is not uncommon for infants with hypocalcemic convulsions,
such as those shown to respond better to Mg therapy than to Ca or
to barbiturates (Review: Seelig, 1978), to be treated with only
Ca, Mg levels never having been obtained. Also, patients with
manifestations of Mg deficiency and recorded low Mg levels (i.e
with alcoholism, cardiac disease, or recovering from surgery)
have been treated conventionally, without Mg repletion.
Adaptation to low Mg may explain the clinical tolerance of
failing to correct Mg deficiency. Long-term follow-up of patients
at risk, with and without Mg repletion, should yield important
information. Intervention studies in older populations, selecting
groups at risk of disorders to which Mg deficiency might be
contributory: hypertensives or cardiacs receiving diuretics,
those with family histories of IHD, and patients with diabetes
mellitus, might provide clues more quickly.
REFERENCES
Adams JH, Mitchell JRA (1979). The effects of agents which
modify platelet behavior and of magnesium ions on thrombus
formation in vivo. Thrombos Haemostas (Stuttgart) 42: 603.
Aikawa JK (1960). Effects of pyridoxine and desoxypyrodixine
on magnesium metabolism in the rabbit. Proc Soc Exp Biol Med
104:461.
Alcock NW, Shils ME (1974). Serum immunoglobulin G in the
magnesium depleted rat. Proc Soc Exp Biol Med 145: 855.
Alcock NW, Shils ME, Lieberman PH, Erlandson RA (1973). Thymic
changes in the magnesium-depleted rat. Cancer Res 33:2196.
Altura BM (1982). Magnesium and regulation of contractility of
vascular smooth muscle. Adv Microcirc 11:77.
Altura BM, Altura BT (1981). Role of magnesium ions in
contractility of blood vessels and skeletal muscles. Magnesium
Bull 3:102.
Altura BT, Altura BM (1980). Withdrawal of magnesium causes
vasospasm while elevated magnesium produces relaxation of tone in
cerebral arteries. Neurosc Letters 20:323.
Altura BN, Altura BT, Carella A, (1981). Hypomagnesemia and
vasoconstriction: possible relationship to etiology of sudden
death ischemic heart disease and hypertensive vascular diseases.
Artery 9:212.
Anderson TW, Neri LC, Schreiber GB, Talbot FDF, Zdrojewski A
(1975). Ischemic heart disease, water hardness and myocardial
magnesium. Canad Mad Assoc J 113:199.
Baker H, Frank O, Jaslow SP (1980). Oral versus intramuscular
vitamin supplementation for hypovitaminosis in the elderly. J Am
Geriat Soc 28:42-45.
Baker H, Frank O, Thind IS, Jaslow SP, Louria D (1979).
Vitamin profiles in elderly persons living at home or in nursing
homes, versus profile in healthy young subjects, J Am Geriat Soc
27:444.
Baker PF, Rink TJ (1975). Catecholamine release from bovine
adrenal medulla in response to maintained depolarization. J
Physiol 253:593.
Baskin SI, Uricchio FJ, Kendrick ZV (1979). The effect of age
on the regional distribution of four cations in the rat heart.
Age 2:64.
Baskin SI, Kuhar KP, Uricchio FJ, Harper GR (1981). The effect
of age on five ions of the kidney in the Fischer 344 rat. Reprod
Nutr Develop 21:689.
Blaxter KL, Wood WA (1952). The nutrition of the young
ayrshire calf. 9. Composition of the tissues of normal and
dystrophic calves. Brit J Nutr 6:144.
Breton A, Walbaum R, Raisnel N (1961). Dosage de la magnesemia
chez 1’ enfant dans diver etats pathologiques. Pediatrie
16:445.
Brierley GP, Jacobus WE, Hunter GR (1967). Ion transport by
heart mitochondria. VIII Activation of ATP supported accumulation
of Mg++ BY Zn++. J Biol Chem 242:2192.
Chadda KD, Lichstein E, Gupta P (1973). Hypomagnesemia and
refractory arrhythmia in a nondigitalized patient. Am J Card
31:98.
Crapo PA (1982). Nutrition in the aged. In Schreier RW (ed):
“Clinical Internal Medicine in the Aged.”
Philadelphia: Saunders, p. 167.
Cummings JR (1960). Electrolyte changes in heart tissue and
coronary arterial and venous plasma following coronary occlusion.
Circ Res 8:865.
Draper HH (1964). Calcium and magnesium metabolism in
senescent mice. J Nutr 83:65.
Durlach J (1981). Clinical aspects of chronic magnesium
deficiency: In Cantin M, Seelig MS (eds): “Magnesium in
Health and Disease.” NY: SP Med Sc Books (2nd Intl Mg Symp
1976): p. 883.
Durlach J (1969). Donnees actuelles; les mecanismes de
synergie entre vitamine B6 et magnesium. J Med
Besancon 5:349.
Durlach J (1967). Le role antithrombosique physiologique du
magnesium. A propos d’une maladie phlebothrombosante par
deficit magnesien. Coeur Med Interne 6:213.
Dyckner T (1980). Serum magnesium in acute myocardial
infarction. Relation to arrhythmias. Acta Med Scan 207:59.
Dyckner T, Wester PO (1978). Intracellular potassium after
magnesium infusion. Brit Med J 1:822.
Dyckner T, Wester PO (1981). Relation between potassium,
magnesium and cardiac arrhythmias. Acta Med Scand Suppl
647:163.
Eckel RH, Hoefeldt FD (1982). Endocrinology and metabolism in
the elderly. In Schrier RW (ed). “Clinical Internal
Medicine in the Aged.” Philadelphia: Saunders, p 222.
Elin RJ (1973). Erythrocyte survival in magnesium-deficient
rats. Proc Exp Biol & Med 142:1159.
Elin RJ (1976/1981). Role of magnesium in membranes;
erythrocyte and platelet function and stability. In Cantin M,
Seelig MS (eds): “Magnesium in Health and Disease.”
New York: SP Med Sci Books (Proc 2nd Intl Mg Symp), p 113.
Elin RJ, Johnson E (1982). The determination of the magnesium
content of blood nononuclear cells. J Am Coll Nutr 1:117.
Exton-Smith AN (1972). Physiological aspects of aging;
relationship to nutrition. Am J Clin Nutr 25:853.
Food & Nutr Board (1980). Recommended Dietary Allowances
Ed. 9, Washington, D.C. National Acad Sci.
Gershoff SN, Prien EL (1967). Effect of daily MgO and vitamin
B6 administration to patients with recurring calcium
oxalate kidney stones. Am J Clin Nutr 20:393.
Go1dsmith LA (1967). Relative magnesium deficiency in the rat.
J Nutr 93: 87.
Goldsmith NF, Go1dsmith JR (1966). Epidemiological aspects of
magnesium and calcium metabolism. Arch Env Health 12:607.
Goldsmith NF, Johnston JO (1976/1981): Magnesium-estrogen
hypothesis: thromboembolic and mineralization ratios. In Cantin
M, Seelig MS (eds). “Magnesium in Health &
Disease.” New York: Sp Med Sc Books (2nd Intl Mg Sympos) p.
313.
Goldsmith NF, Pace N, Baumberger JP, Ury H (1970). Magnesium
and citrate during the menstrual cycle: Effect of an oral
contraceptive on serum magnesium. Fertility Sterility 21:292.
Goulding A, McChesney R (1977). Oestrogen-progestogen oral
contraceptives and urinary calcium excretion. Clin Endocr
6:449.
Greenberg DM (1939). Mineral metabolism: calcium magnesium and
phosphorus. Ann Rev Biochem 8:269.
Geunounou M, Armier J, Gaudin-Harding F (1978). Effect of
magnesium deficiency and food restriction on the immune response
in young mice. Intl J Vit Nutr Res 48:90.
Gueux E, Rayssiguier Y (1981). The hypercholesterolaemic
effect of magnesium deficiency following cholesterol feeding in
the rat. Magnesium Bull 2:126.
Gunther T (1981). Biochemistry and pathochemistry of
magnesium. Artery 9:167.
Hall DA (1969). Connective tissues. In Bakerman (ed):
“Aging Life Processes.” Springfield, CT Thomas, p.
79.
Hamfelt A (1969). Age Variation of vitamin B6
metabolism in man. Clin Chim Acta 10:48.
Harman D, Heidrick ML, Eddy DE (1977). Free radial theory of
aging: effect of free-radical-reaction inhibitors on the immune
response. J Am Geriatr Soc 25:400.
Hass GM, McCreary PA, Laing GH (1976/1981). Lymphproliferative
and immunologic aspects of magnesium deficiency. In Cantin M,
Seelig MS (eds). “Magnesium in Health and Disease.”
NY: Sp Med Sci Books, P. 185 (2nd Intl Mg Symp, 1976).
Heath H (1971). Platelet adhesiveness and aggregation in
relation to diabetic retinopathy. Diabetologia 7:308.
Hellerstein EE, Nakamura M, Hegsted DM, Vitale JJ (1960).
Studies on the interrelationships between dietary magnesium,
quality and quantity of fat, hypercholesterolemia and lipidosis.
J Nutr 71:339.
Henrotte JG Benech A, Pineau M (1976/1981). Relationships
between blood magnesium content and age in a French population.
In Cantin M, Seelig MS (eds): “Magnesium in Health and
Disease.” New York: SP Med Sci Books, (2nd Intl Mg Sympo).
p 929.
Heroux O, Peter D, Heggtveit A (1977). Long-term effect of
sub-optimal dietary magnesium and calcium contents of organs, on
cold tolerance and on life span, and its pathological
consequences in rats. J Nutr 107:1640.
Hochrein H, Kuschke HJ, Zaqqa Q, Fahl E (1967). Das Verhalten
der Intracellularen Magnesium-konzentration in Myokard bei
Insuffizienz, Hypoxie und Kammerflimmern. Klin Wschr 45:1093.
Hsu JM (1965). Zinc content in tissues of pyridoxine deficient
rats. Proc Soc Exp Biol Med 119:177.
Hungerford GF, Karson HF (1960). Eosinophilia of magnesium
deficiency. Blood 16:1642.
Hunt SM, Schofield FA (1969). Magnesium balance and protein
intake level in adult human female. Am J Clin Nutr 22:367.
Irwin MI, Feeley RM (1967). Frequency and size of meals and
serum lipids, nitrogen and mineral retention in young women. J
Clin Nutr 20:816.
Ising H (1981). Interaction of noise-induced stress and Mg
decrease. Artery 9:205.
Itokawa Y, Tseng LF, Fujiwara M (1974). Thiamine metabolism in
magnesium-deficient rats. J Nutr Sci Vit 20:249.
Jackson CE, Meier DW (1968). Routine serum Mg analysis. Ann
Intern Med 69:743.
Jasmin G (1969). Lymphoedeme, hyperplasie et tumefaction du
tissu lymphatique chez le rat soumis a une diete deficiente en
magnesium. Rev Can Biol 22:383.
Jennings RB, Shen AC (1972). Calcium in experimental
myocardial ischemia. In Bajusz E, Rona G (eds): “Recent
Advances in Studies on Cardiac Structure and Metabolism I.”
Myocardiology. p 639.
Johansson G (1979). Magnesium metabolism. Studies in health,
primary hyperparathyroidism and renal stone disease. Scand J Urol
Nephrol. Suppl 51:1
Karppanen H, Pennanen R, Passinen L (1978). Minerals and
sudden coronary death. Adv Card 25:9.
Keating FR, Jr., Jones JD, Elveback LR, Randall RV (1969). The
relation of age and sex to distribution of values in healthy
adults of serum calcium, inorganic phosphorus, magnesium,
alkaline phosphatase, total proteins, albumin, and blood urea. J
Lab Clin Med 73:825.
Keating MJ, Sethi MR, Bodey GP, Samaan NA (1977). Hypocalcemia
with hypoparathyroidism and renal tubular dysfunction associated
with aminoglycoside therapy. Cancer 39:1410.
Larvor Magnesium, humoral immunity and allergy. In Cantin M,
Seelig MS (eds). “Magnesium in Health and Disease" NY: Sp
Med Sci Books, p 201 (2nd Intl Symp on Mg).
Lennon J, Lennon J, Jr., Piering WF, Larson L (1974). Effect
of glucose on urinary cation excretion during chronic
extracellular volume expansion in normal man. J Clin Inv
53:1424.
Lifshitz F, Harrison HC, Harrison HE (1967a). Response to
vitamin D of magnesium deficient rats. Proc Soc Exp Biol Med
125:472.
Lifshitz F, Harrison HC, Harrison HE (1976b). Effects of
vitamin D on magnesium metabolism in rats. Endocrinology
81:849.
Lindeman RD (1976/1981). Nutritional influences on magnesium
homeostasis with emphasis on renal factors. In Cantin M, Seelig
MS (eds). “Magnesium in Health and Disease.” NY: SP
Med and Sci Books, p 381 (Proc 2nd Intl Mg Symp).
Lindeman RD, Adler S, Yiengst MJ, Beard ES (1967). Influence
of various nutrients on urinary divalent cation excretion. J Lab
Clin Med 70:236.
Makinodan T, Yunis E (1977) (eds). “Immunology and
Aging.” New York: Plenum Medical Book Co.
Martin HE, Mehl J, Wertman M (1952). Clinical studies of
magnesium metabolism. M Clin North America 36: 1157.
McCance RA, Widdowson EM, Lehmann H (1942). Effect of protein
intake on absorption of calcium and magnesium. Biochem J
36:686.
McCoy JH, Kenney MA (1975). Depressed immune response in
magnesium deficient rat. J Nutr 105:791.
McGandy RB, Barrows CH, Spanias A, Meredith A, Stone JL,
Norris AH (1966). Nutrient intakes and energy expenditure in men
of different ages. J Gerontol 21:581.
McNair P, Christiansen C (1978). Hypomagnesemia: risk factor
in diabetic retinopathy. Diabetes 27:1075.
Medalle R, Waterhouse C, Hahn TJ (1976). Vitamin D resistance
in magnesium deficiency. Am J Clin Nutr 29:854.
Melnick I, Landes RR, Hoffman AA, Burch JF (1971). Magnesium
therapy for recurring calcium oxalate urinary calculi. J Urol
105:119.
Miller ER, Ullrey DE, Zutaut CL, Baltzer By, Schmidt DA,
Vincent BH, Hoefer JA, Luecke RW (1964). Vitamin D2
required of the baby pig. J Nutr 83:140.
Moles KW, McMullen JK (1982). Insulin resistance and
hypomagnesaemia: case report. Br Med J 285:262.
Mori K, Duruisseau J (1960). Water and electrolyte changes in
aging process with special reference to calcium and magnesium in
cardiac muscle. Can J Bioch Physiol 38:919.
Mostow SR (1982). Infectious diseases in the aged. In Schrier
RW (ed). “Clinical Internal Medicine in the Aged.”
Philadelphia: Saunders, p 256.
Mountokalakis TH, Singhellakis PN, Alevizaki CC, Caramanakos
E, Ikkos DG (1976). Absorption intestinale du magnesium chez des
malades en insuffisance renale chronique. Rev Franc Endocr Clin
Nutr Metab 17:229.
Munro HN, Young VR (1978). Protein metabolism in the elderly.
Postgrad Med 63:143.
Nayler WG (1967). Calcium exchange in cardiac muscle: A basic
mechanism of drug action. Am Heart J 73:379.
Neri LC, Marier JR (1978/1982). Epidemiology of sudden cardiac
death — minerals and water story. In Naito HK (ed).
“Nutrition and Heart Disease.” New York: SP Med Sci
Books, p 81.
Nuoranne PJ, Raunio RP, Saukko P, Karppanen H (1980).
Metabolic effect of a low magnesium diet in pigs. Br J Nutr
44:53.
Oldham HG (1962). Thiamine requirements of women: Ann NY Acad
Sci 98:542.
Parisi AF, Val1ee BL (1969). Zinc metalloenzymes:
characteristics and significance in biology and medicine. Am J
Clin Nutr 22:1222.
Paunier L, Radde IC, Kooh SW, Conen PE, Fraser D (1968).
Primary hypomagnesemia with secondary hypocalcemia in an infant.
Pediatrics 41:385.
Prouvost-Danon A, Larvor P, Rayssiguier Y, Wyczolkowska J,
Durlach J (1975) . Taux serique d’anticorps reaginiques
(IgE) chez la souris en carence magnesique. Rev Fr Allerg
15:147.
Rayssiguier Y (1981). Magnesium and lipid interrelationships
in the pathogenesis of vascular diseases. Magnesium Bull
3:165.
Rayssiguier Y, Badinand F, Kopp J (1979). Effects of magnesium
deficiency on paturition and uterine involution in the rat. J
Nutr 109:2117.
Reddy V, Sivakumar B (1974). Magnesium-dependent vitamin
D-resistant rickets. Lancet 1:963.
Reinhold JG, Bahram F, Parichehr A, Ismail-Beigi F (1976).
Decreased absorption of calcium, magnesium, zinc and phosphorus
consumption as wheat bread. J Nutr 106:493.
Rigo J, Szelenyi I, Sos J (1967). Connection between vitamine
B6 and magnesium balance. Acta Physiol Acad Sc Hung
32:16.
Rosler I, Rabinowitz D (1973). Magnesium-induced reversal of
vitamin D resistance in hypoparathyroidism. Lancet I: 803.
Ross PS, Seelig MS, Berger AR (1976/1981). Isolation of
leukocytes for magnesium determination. In Cantin M, Seelig MS
(eds). “Magnesium in Health and Disease.” New York:
SP Sc Books, p 7 (Proc 2nd Intl sympo).
Ross PS, Seelig MS, Berger AR (1982). Pilot studies of mixed
white cells and lymphocytes. J Am Coll Nutr 1:118.
Ryan MP, Ryan MF, Counihan TB, Thornton L (1981). The use of
lymphocytes to monitor cellular magnesium and potassium.
Magnesium Bull 2:113.
Savoie LL (1972). Production de necroses cardaques
non-oclusives chez le rat par un regime thrombogeer. Path Biol
20:117.
Savoie LL, DeLorme B (1976/1981). Magnesium .inhibition of
cardiac lesions produced by sodium phosphate in hyperlipemic
rats. In Cantin M, Seelig MS (eds). “Magnesium in Health
and Disease.” New York: SP Med Sci Books, p 57 (2nd Intl Mg
symp)
Sawyer M, Baumann L, Stevens F (1918). Studie of acid
production. The mineral loss during acidosis. J Biol Chem
33:103.
Schwartz R, Walker G, Linz MD, MacKellar I (193). Metabolic
responses of adolescent boys to two levels of dietary magnesium
and protein. I. Magnesium and Nitrogen Retention. Am J Clin Nutr
26:510.
Schwartz K (1962). Vitamin E trace elements and sulfhydryl
groups in respiratory decline. Vits Horm 20:3.
Seelig MS (1977/1982). Early nutritional root of
cardiovascular disease. In Naito HK (ed). “Nutrition and
Heart Disease.” New York: SP Sci Med Books, p 31 ( Proc
19th Ann Mtg-Nutr).
Seelig MS (1979). Magnesium (and trace substance) deficiencies
in the pathogenesis of cancer. Biol Tr Elem Res 1:273.
Seelig MS (1980). “Magnesium Deficiency in the
Pathogenesis of Disease. Early Roots of Cardiovascular, Skeletal,
and Renal Abnormalities.” New York: Plenum Publishing
Corp.
Seelig MS (1978). Magnesium deficiency with phosphate and
vitamin D excesses: role in pediatric cardiovascular disease?
Cardiovasc Med 3:637.
Seelig MS (1972). Myocardial loss of functional magnesium II .
In Cardiomyopathies of Different Etiology. In Bajusz E, Rona G
(eds). “Recent Advances in Studies on Cardiac Structure and
Metabolism. I. Myocardiology.” Baltimore, Univ Park Press,
p 626.
Seelig MS (1981). Magnesium requirements in human nutrition.
Magnesium Bull 3:26.
Seelig MS (1980/1983). Nutritional roots of combined systemic
disorders. In Lifshitz F (ed). New York: Marcel Dekker Inc. (in
press).
Seelig MS (1964). The requirement of magnesium by the normal
adult. Am J Clin Nutr 14:342.
Seelig MS (1969). Vitamin D and cardiovascular, renal and
brain damage in infancy and childhood. Ann NY Acad Sci
147:537.
Seelig MS. Berger AR, Avioli LA (1976). Speculations on renal,
hormonal and metabolic aberrations in a patient with marginal
magnesium deficiency. In Cantin M, Seelig MS (eds).
“Magnesium in Health and Disease.” New York: SP Med
Sci Books, p 459.
Seelig MS, Haddy FJ (1976/1981). Magnesium and the arteries I.
Effects of magnesium deficiency on arteries and on the retention
of sodium, potassium, and calcium. In Cantin M, Seelig MS (eds).
“Magnesium in Health and Disease.” New York: SP Med
Sc Books, p 605 (Proc 2nd Intl Mg Symp).
Seelig MS, Heggtveit HA (1974). Magnesium interrelationships
in ischemic heart disease: A review. Am J Clin Nutr 27:59.
Shils ME (1962). Metabolic aspects of tetracyclines. Clin
Pharm and Ther 3:321.
Simpson FO, Nye ER, Bolli P, Waal-Manning HJ, Goulding AW et
al (1978). The Milton survey: Part 1. General methods, height,
weight and 24 hour excretion of sodium, calcium, magnesium, and
cretinine. New Zeal Med 3 87:379.
Slavin JL, Marlett JA (1980). Influence of refined cellulose
on human bowel function and calcium and magnesium balance. Am J
Clin Nutr 33:1932.
Stendig-Lindberg G (1972). Hypomagnesemi och uppkomst av
alkoholence-alopatier. Lakartingningen 69:1237.
Szelenyi I, Rigo R, Ahmen BO, Sos J (1967). The role of
magnesium in blood coagulation. Thromb Diath Haemorrh 18:
626.
Tappel AL (1968). Will antioxidant nutrients slow aging
processes? Geriatrics 23:97.
Touitou Y, Touitou C, Bogdan A, Beck H, Reinberg A (1978).
Serum magnesium circadian rhythm in human adults with respect to
age, sex, and mental status. Clin Chim Acta 87:35.
Turlapaty PDVM, Weiner R, Altura BM (1981). Interactions of
magnesium and verapamil on tone and contractility of vascular
smooth muscle. Europ J Pharmacol 74:263.
Uauy R, Scrimshaw N, Young V (1978). Human.protein
requirements: nitrogen balance response to graded levels of egg
protein in elderly men and women. Am J Clin Nutr 31:779.
US Dept Agric Nationwide Food Consumption Survey, 1977-1978
(1980). USDA Sci & Educ Adm.
US Senate Select Comm on Nutr & Human Needs (1978).
Dietary goals for the U.S. Govt Printing Off. Washington,
D.C.
Vallee BL (1960). Metal and Enzyme interactions: Correlation
of Composition, Function and Structure. The Enzymes 3:225.
Vir SC, Love AHG (1979). Nutritional status of
institutionalized aged in Belfast, Northern Ireland. Am J Clin
Nutr 32:1934.
Vitale JJ, White PL, Nakamura N, Hegsted DM, Zamcheck N,
Hellerstein EE (1957). Interrelationships between experimental
hypercholesteremia magnesium requirement, and experimental
atherosclerosis. J Exp Med 106:757.
Wacker WEC (1980). “Magnesium and Life.”
Cambridge:Harvard Univ Press.
Wallach S (1976/1981). Physiologic and critical interrelations
of hormones and magnesium. Consideration of thyroid, insulin,
corticosteroids, sex steroids and catecholamines. In Cantin M,
Seelig MS (eds). “Magnesium in Health and Disease.”
New York: SP Med Sc Books, p 241 (2nd Intl Mg Symp).
Walser M (1967). Magnesium metabolism In Review Physiol
Biochem Exp Pharm 59:185.
Young GA, Parsons FM (1977). Effects of dietary deficiencies
of magnesium and potassium on growth and chemistry of
transplanted tumors and host tissues in the rat. Europ J Cancer
13:103.
Zieve FJ Freude KA, Zieve L (1977). Effects of magnesium
deficiency on protein and nucleic acid synthesis in vivo.J Nutr
107:2178.
Zieve L, Doizaki WM, Stenroos LB (1968). Effects of magnesium
deficiency on growth response to thiamine of thiamine-deficient
rats. J Lab Clin Med 72:261.
Zunkley I, Schmidt PF, Vetter H, Elies M (1982). Determination
of magnesium concentration in rbc by laser-micro-mass analysis
(LAMMA). J Am Coll. Nutr 1: (in press).
This page was first uploaded to The Magnesium Web Site on
October 11, 2002
http://www.mgwater.com/










