In: Magnesium in Health and Disease, Y Itokawa and J.
Durlach, Eds., John Libbey Press, London, Paris, 1989;
565-571
Magnesium, Water Hardness, and Heart Disease
T.W. Anderson
W.H. Leriche
D. Hewitt
L.C. Neri
The belief that the mineral content of drinking water may
influence cardiovascular death rates has attracted a great deal
of attention since Kobayashi (1957) and Schroeder (1960)
published their original observations.
It remains possible that some of the correlations that have
been observed between death rates and water hardness are nothing
more than statistical artefacts, but this discussion will be
restricted to the Province of Ontario, where the evidence in
favor of a cause-and-effect relationship is reasonably strong,
and where magnesium (Mg) appears to be the substance
responsible.
Ontario covers a large area, stretching approximately 1000
miles in both north-south and east-west directions. In the north
the water is very soft (i.e. low in minerals) and generally
contains no more than 2-3 mg of Mg/per liter, while in the
extreme south there is an area with water that is very hard due
to high concentrations of both calcium (Ca) and Mg. Here the
drinking water may contain over 100 mg/per liter of Ca, and up to
40 mg/per liter of Mg. Since the average North American diet
supplies about 200 mg of Mg per day (Schroeder, 1969), an intake
of 1-2 liters of some of these southern Ontario water supplies
could therefore increase daily intake by as much as 20-40%, which
could be crucially important, if, as Seelig (1964) has claimed,
the usual dietary intake of Mg is barely adequate.
In our studies we have attempted to identify which of the
several links in the chain of events leading to death from heart
attack is responsible for the increased death rate seen in the
soft-water areas; a second line of attack has been to look for
substance in the blood or tissues that shows a variation in
concentration across the province parallel to that seen in local
water supplies.
Our first study (Anderson et al., 1969) was prompted
by the observation (Crawford and Crawford, 1967) that despite a
large difference in the death rates from heart disease in Glasgow
(soft water) and London (hard water), the frequency of coronary
atherosclerosis was similar in both places. Since the
excitability of muscles and nerves is known to vary with the
concentration of Ca and Mg (the major "hardness" minerals), we
examine the Ontario data for evidence of an excess of sudden
deaths (due to abnormal electrical rhythms) in the soft-water
areas.
We divided the province into three areas of water hardness,
less than 100 ppm, 100-200 ppm, and greater than 200 ppm, and
examined mortality data over a 3-year period, 1965-1967. The only
index of sudden death available on the official data cards was
whether the death had been certified by a coroner, rather than by
a private physician. Having first established that there was
little or no gradient across the three areas in the proportion of
noncardiac deaths certified by coroners. we examined
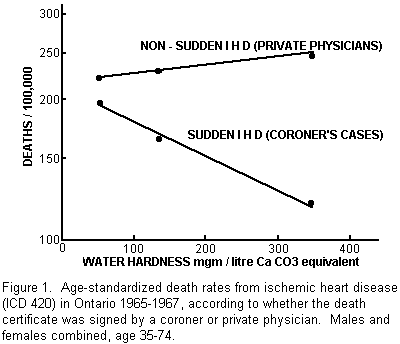
ischemic heart disease (IHD) deaths and found that there was
indeed a steep gradient, with sudden IHD death rates being almost
twice as high in the soft-water area as in the hard-water area
(Fig. 1).
We could find no evidence that this excessive sudden-death
rate was related to the more severe winters in the soft
(northern) part of the province (Anderson and La Riche, 1970) or
to a number of socioeconomic variables (Anderson and La Riche,
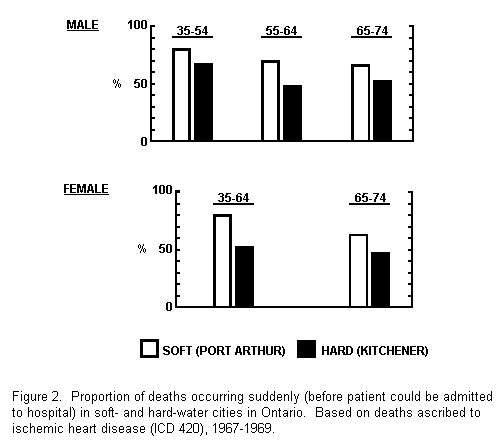
1971). Furthermore, a detailed investigation of all deaths
occurring over a 3-year period in two cities near the extremes of
the water hardness spectrum, confirmed that the proportion of IHD
deaths occurring suddenly was higher in the soft-water town
(Anderson and Le Riche. 1971), and the difference was apparent in
both sexes and at all ages (Fig. 2).
BLOOD AND TISSUES STUDIES
If a cardiac water factor really exists, it is presumably
something beneficial in hard water or something toxic in soft
water. In either case, one might expect to find evidence of a
difference in blood or tissue levels of the responsible substance
between residents of soft and hard-water areas. The work of
Crawford and Crawford (1967) and Bierenbaum et al.
(1973) encouraged us to believe that the link was more likely to
a beneficial hard-water factor (probably Ca. but possibly Mg)
than a toxic soft-water factor, and we therefore arranged for
serum samples to be collected from middle-aged residents in two
Ontario cities, one with very hard water, the other with very
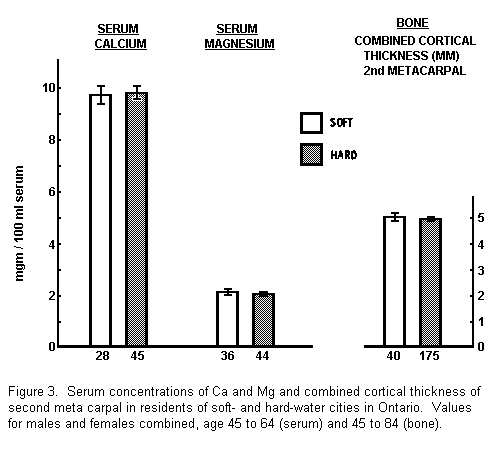
soft water, and for the samples to be analyzed for Ca and Mg. In
addition we compared x-rays of the hand in the two areas to see
if there was any difference in the degree of bone mineralization.
The findings in this study were entirely negative (Fig. 3); there
were identical mean serum levels for both Ca and Mg, and no
difference in the x-ray measurements.
Up to this time we had favored Ca as the more likely
protective element of the major "hardness" cations. However,
since the differences in serum levels were negligible,
intracellular concentrations might be more important,
and Mg is a much more plentiful ion within the cell (particularly
in heart muscle) than is Ca. We had also become aware of Seelig's
evidence (1964) that Western diets are barely adequate in Mg, so
that the relatively small water-borne intake might be crucial.
The findings of Heggtveit et al. (1969) that the
myocardium was low in Mg in persons dying of heart attacks also
lent strength to the idea that Mg, rather than Ca, was a more
likely candidate for the Ontario "water factor."
We therefore arranged with pathologists in a number of
softand-hard-water cities in Ontario for a portion of heart
muscle to be retained from each routine autopsy performed over a
4-month period (Anderson et al., 1975). The apex of the
heart was examined in each case. This was done to ensure
uniformity of sampling site and to decrease the likelihood of
obtaining an area of very recent (and therefore inapparent)
myocardial infarction.
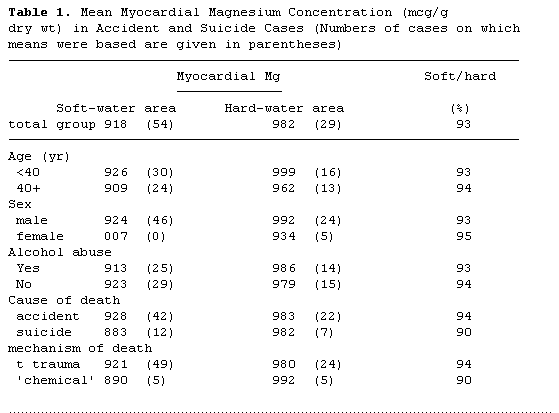
Myocardial samples were obtained from 54 violent deaths in 5
soft-water cities, and from 29 violent deaths in 3 hard-water
cities. The mean Mg concentration was 918 mcg per g (dry tissue)
in the soft-water residents, some 7% lower than the mean of 982
mcg per g in the hard-water residents. This difference was
statistically significant at P < 0.01. No significant
differences were seen in the concentration of Ca (soft 224, hard
232 mcg per g), zinc (103, 102), copper (15.7, 16.4). chromium
(0.9, 0.13), or cadmium (0.14, 0.14). Lead was detectable at a
level of 0.01 mcg per g or greater in only 26% of the cases in
the soft- water area, and 21% in the hard-water area.
No significant differences were found in the distribution
according to sex, age, or history of alcohol abuse in the softand
hard-water areas (Table 1). Similarly, suicides, as well as
accidental deaths, and traumatic, as well as "chemical" deaths
(e.g. drug overdose or carbon monoxide poisoning), showed
essentially the same geographical differences.
Samples of diaphragm and pectoralis major muscles were also
analyzed, as examples of another continuously active muscle and a
typical skeletal muscle. Neither of these muscles showed a
statistically significant difference in Mg concentration between
the two areas.
Samples were also obtained from a total of 40 IHD deaths. As
previously reported by Heggtveit et al. (1969), the
myocardial Mg levels were much lower than in the accident cases,
with mean values of 697 and 744 mcg per g in the soft- and
hard-water areas, respectively; over 20% below the corresponding
values in accidental deaths.
Since these results were published, an apparently
contradictory geographic pattern has been reported from England
(Chipperfield et al., 1976). In this study, heart muscle
samples from non-cardiac deaths were compared from two cities,
Hull (hard water) and Burnley (soft water), and a 19% difference
found in the "wrong" direction. However, by Ontario standards the
Mg content of both water supplies was very low (Hull 5 and
Burnley 2 mg per liter), and since the difference between them
represents barely 2% of probable dietary intake, water is
unlikely to make any difference to total supply. (Magnesium
levels in most British water supplies are relatively low compared
to Ontario, so that if there is a real "water factor" in Britain
it is probably something other than Mg.) The reason for the
differences in myocardial Mg reported by Chipperfield et
al. is unclear, but some procedural difference is a distinct
possibility, since an even larger difference (37%) was found in
their myocardial potassium concentrations.
CONCLUSION
At least some of the geographical variation in heart disease
mortality in Ontario may be related to a marginally inadequate
dietary intake of Mg. In the hard-water area of the province,
water-borne Mg increases total daily intake by at least 20% so
that residents of this area are less likely to be Mg deficient.
This hypothesis is supported by the finding that the
concentration of Mg in the myocardium tends to be higher in
residents of the hard-water area, and it is consistent with the
observation that fatal cardiac arrhythmias are less common than
in the soft-water area.
SUMMARY
Death rates from IHD in the Province of Ontario show an
inverse correlation with hardness of local water supply, due to
an excess of sudden deaths in the soft-water area. An autopsy
comparison of accident victims in the soft- and hard-water areas
showed that the mean myocardial Mg concentration was 7% lower
(P < 0.01) in the soft-water area. No significant
geographical differences were seen in Mg concentrations in
diaphragm or pectoral muscles, or (in previous studies) serum
concentrations or skeletal mineralization. The autopsy comparison
also confirmed the previously reported low levels of myocardial
Mg in victims of heart attacks. It would appear that, in Ontario
at least, the contribution of water-borne Mg to total dietary
intake may be critical, and that some residents of soft-water
areas are in a state of subclinical Mg deficiency. If these
individuals suffer a myocardial infarction, they may then be at
an increased risk of developing a fatal cardiac arrhythmia,
leading to sudden death.
REFERENCES
Anderson. T.W., Le Riche, W.H.. and MacKay. J.S. Sudden death
and Ischemic heart disease: Correlation with hardness of local
water supply. New England J. of Med. 280, 805-807
(1969).
Anderson, T.W. and Le Riche, W.H. Cold weather and myocardial
infarction. Lancet, 291-296 (1970).
Anderson, T.W. and Le Riche, W.H. Sudden death from ischemic
heart disease in Ontario and its correlation with water hardness
and other factors. Can. Med. Assoc. J. 105, 155-160
(1971).
Anderson, T.W. Serum electrolytes and skeletal mineralization
in hard and soft-water areas. Can. Med. Assoc. J. 107,
36-37 (1972).
Anderson, T.W., Neri, L.C., Schreiber, G.B., Talbot, F.D.F.,
and Zdrojewski, A. Ischemic heart disease, water hardness and
myocardial magnesium. Can. Med. Assoc. J. 113, 199-203
(1975).
Bierenbaum, M.L., Fleischman, A.I., Dunn, J.P., Hayton, T.,
Pattison, D.C., and Watson, P. Serum parameters in hard and soft
water communities. Am. J. Public Health 63, 169-173
(1973).
Chipperfield, B., Chipperfield, J.R., Behr, G., and Burton, P.
Magnesium and potassium content of normal heart muscle in areas
of hard and soft water. Lancet 7, 121-123 (1976).
Crawford, T. and Crawford. M.D. Prevalence and pathological
changes of ischemic heart-disease in a hard-water and in a
soft-water area. Lancet 1, 229-232 (1967).
Duruisseau, J. P., Lapointe, J., and Laurendeau, E. Teneur en
magnésium de la diète servie à
l'Hôpital Notre-Dame de la Merci.. Can. J. Public Health
67, 30-32 (1976).
Heggtveit, H.A., Tanser, P., and Hunt, B. Magnesium content of
normal and ischemic hearts. 7th International. Congress. of
Pathol., Montreal, July 13-19 (1969), p. 53.
Kobayashi, J. On geographical relationship between the
chemical nature of river water and death-rate from apoplexy.
Bar. Ohara Inst. Landwirt. Biol. 11, 12-21 (1957).
Schroeder, H.A. Relation between mortality from cardiovascular
disease and treated water supplies. J. Am. Med. Assoc.
172. 1902-1908 (1960).
Schroeder, H.A., Nason, A.P., and Tipton, I.H. Essential
metals in man: Magnesium. J. Chronic Dis. 21, 815-841
(1969).
Seelig, M.S. The requirement of magnesium by the normal adult.
Am. J. Clin. Nutr. 14, 342-390 (1964).
This page was first uploaded on June 19, 1996
http://www.mgwater.com/




