The Science of the Total Environment, 42 (1985)
49-75
Elsevier Science Publishers B.V., Amsterdam
MAGNESIUM AND CERTAIN OTHER ELEMENTS AND CARDIOVASCULAR
DISEASE
L.C. NERI1, H.L. JOHANSEN2, D.
HEWITT3, J. MARIER4, AND N.
LANGNER1
1Department of Epidemiology, University of Ottawa,
451 Smyth Road, Ottawa, K1H 8M5 (Canada)
2Centre for Disease Control, Health and Welfare,
Tunney’s Pasture, Ottawa, K1A 0L2 (Canada)
3Department of Community Health, University of
Toronto, 12 Green Park Crescent West, Toronto, M5S 1A8
(Canada)
4Secretariat, National Research Council, 100 Sussex
Drive, Ottawa, K1A 0R6 (Canada)
ABSTRACT
Cardiovascular disease (CVD) continues to be the major cause
of mortality in developed countries. For the past two-and-a-half
decades the inverse relationship between water hardness and CVD
mortality has stimulated interest among epidemiologists,
clinicians and experimental researchers. Much progress has been
made in elucidating which element in the water may account for
this situation.
After reviewing those elements found to have a role in
cardiovascular function the authors present the epidemiological
evidence and its consistency with recent findings: aside from
various trace elements emphasis is placed on magnesium which is
recognized as having a vital role.
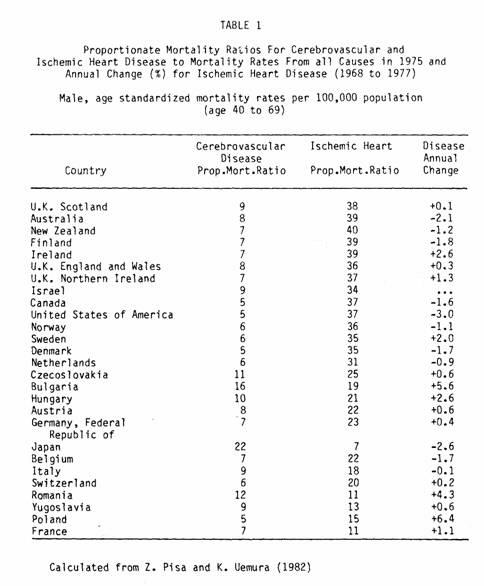
Cardiovascular disease (CVD) is the leading cause of death
among men in most industrialized countries, accounting for 37-56%
of deaths in 27 countries studied by the World Health
Organization (Table 1) (1). Ischemic heart disease (IHD) alone
accounts for up to 24% of mortality from all causes in males aged
40-69 despite the decline in death rates from heart disease
observed since the mid-1960’s in several countries. There
is no fully satisfactory explanation for this recent downward
trend, just as there was none for the upward trend prior to the
mid-1960s. Factors which might explain this downward trend
include a decrease in mortality from and/or a decrease in the
incidence of disease. The former could arise from improved
treatment such as coronary bypass surgery, coronary care units,
and anti-arrhythmic medication, whereas a decrease in incidence
could occur as a result of reduction of risk factors through
better treatment of hypertension, changing dietary patterns, and
lower prevalence of smoking.
Over the last decade a number of very large intervention
trials (e.g., MRFIT (2), Oslo Study (3), North Karelia (4),
European Collaborative Group (5) have been conducted to discover
whether people can be persuaded to change their eating and living
habits, and if they do, whether they will be less liable to heart
attacks and live longer. Although the evidence tends to favor
answering both these questions in the affirmative, it is not
entirely clear. Reduction of risk factors was only achieved in a
minority of the study populations, and in fact in some of the
trials, was achieved to almost the same extent in the control
populations. This type of result made it more difficult to answer
the life expectancy question and also poses a further question,
namely, which of the interventions were responsible for the risk
factor reductions?
Trials of this sort are extremely expensive. They require
costly equipment, highly specialized manpower and much labour and
time to attain high compliance rates. The widespread adoption of
the primary prevention techniques employed in these trials would
similarly be extremely costly and questionably effective.
Compare this to the prevention of goiter by the introduction
of iodized table salt. In the 1950 the prevalence of goiter was
high in the low iodine region around the Great Lakes of North
America and in much of Europe (6). Death rates were also high and
in Ontario were twice as high for natives as compared to
immigrants (Fig. 1).
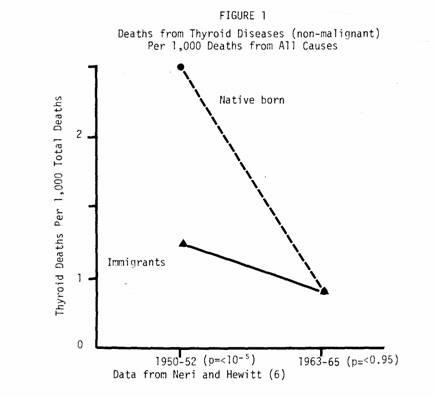
Fifteen years later, iodine in table salt had obliterated
these differences and led to a general decline in mortality.
Thus, here we have a disease causing considerable morbidity
and mortality during middle age, which is attributable to mineral
intake both during early life and in the more recent past.
Furthermore, the mineral content of local drinking water provided
an index of total intake. With the identification of the mineral
came a low cost, highly effective intervention.
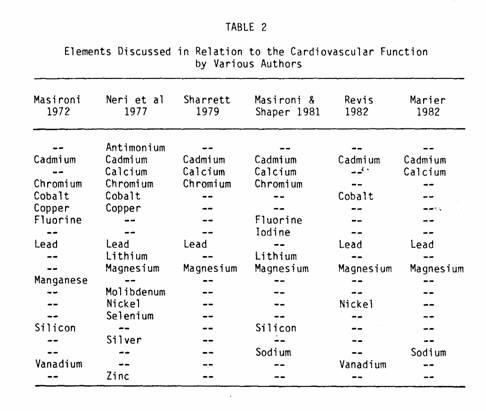
The possibility of an analogous situation occurring with
ischemic heart disease has intrigued investigators and the
cost-effectiveness of this type of primary prevention has
motivated the search for elements which could be involved in the
complex enzymatic and conduction systems in the myocardium (Table
2).
Improvements in chemical analytical techniques have in the
last few decades permitted the study of the role of a number of
elements in various aspects of cardiac function.
We propose to discuss the suggested roles and physiologic
action of these elements, then look at the epidemiologic evidence
in terms of specific hypotheses. We arbitrarily classify the
elements according to the completeness of our knowledge of their
role in cardiovascular function.
TRACE ELEMENTS FOR WHICH THERE IS LITTLE EVIDENCE OF A ROLE
IN CARDIOVASCULAR FUNCTION
Silver
The only evidence that silver plays a role in cardiovascular
disease is that increased concentrations of silver have been
found in the aortas of atherosclerosis victims (7).
Molybdenum
Although there is no direct evidence that molybdenum is
essential in man, its presence as a component of several enzymes
suggests that it may be. It is rapidly excreted, and there is
very little body storage. Tissue determination on myocardial
infarction victims has shown increased molybdenum levels in the
serum and decreased levels in the injured heart tissue (8).
Nickel
Nickel is one of the relatively non-toxic trace metals found
in human tissues (unless inhaled in a form such as nickel
carbonyl). Very few physiological or biological studies of this
element have been done, Experimental evidence of nickel’s
essential role has been obtained in the chick and the rat,
suggesting that nickel may be essential for man (9). However, due
to the extremely small amount needed, it is probably not a
practical consideration.
Plasma nickel levels increase sharply following myocardial
infarction, both in man and in experimental animals, but is not a
specific effect (7,10,11,12,13).
Vanadium
Revis (14) has reviewed the physiologic experimental evidence
associating vanadium with cardiovascular function. Vanadium
inhibits myocardial sarcolemmal NA+ and K+-ATPase, produces a
positive inotropic effect, and stimulates adenylate cyclase.
However, endogenous vanadium levels in the heart are lower than
would be required for the latter two effects, making it difficult
to postulate a physiological role for this element. Epidemiologic
evidence is also slight, but two studies have found negative
correlations between vanadium and atherosclerotic heart disease
(15,16).
Cobalt
Cobalt is physiologically active in man only when supplied in
one particular form: cyanocobalamin or Vitamin B12. Man is
dependent upon the food chain for his supplies of Vitamin B12 as
he has no ability to incorporate cobalt into the vitamin by
himself. When taken in small amounts over a long period of time
and under certain conditions, (e.g., a state of malnutrition and
especially if in an alcoholic vehicle) cobalt may induce
cardiomyopathy (10). There were several cases of severe and
lethal cardiomyopathy in men drinking excessive amounts of beer
in Canada, until breweries stopped adding cobalt to beer
(17,18).
Cobalt inhibits pyruvate and fatty acid oxidation and induces
hyperlipemia (19). These effects can be prevented by Vitamin E
and selenium (20).
Lithium
Lithium is not an essential nutrient for man as far as is
known. However, it has been used pharmacologically in the
treatment of manic depressive psychosis for more than twenty
years.
The evidence for a role in cardiovascular function comes from
Voors who found an inverse correlation between the amount of
lithium in drinking water and cardiovascular disease (15,21).
Other authors have also reported findings that point to a
protective effect of lithium against heart disease (22,23,24).
This association could be accounted for by lithium's protective
effect against several IHD risk factors. However, lithium's
concentration in drinking water is very low, so any effect it
could have would be minimal (15,25).
Selenium
While selenium is considered an essential element in animals,
evidence for a role in human nutrition is very scarce. Its
essential role in man would be in the seleno-enzyme glucation
peroxidose. However, the fact that selenium deficiency in animals
can manifest itself in a wide variety of symptoms provides ample
opportunity for speculation as to its effect on humans. Diseases
postulated to be related to selenium deficiency range from cancer
to dental caries (26).
The amounts of selenium needed to prevent deficiency are
inversely related to dietary levels of Vitamin E. This fact
indicates a co-factor role for selenium (27). Both deficiency and
excess of selenium can lead to myocardial necrosis, elevation of
serum transaminases and degeneration of vascular endothelium
(28). However, the evidence of an effect in humans is still
almost exclusively indirect and comes from epidemiologic
observations. In the United States inverse correlations have been
found between death rates for cardiovascular disease and mean
levels of selenium in blood banks of 19 states (29). In
selenium-deficient regions of China, there is a high prevalence
of Keshan disease affecting young women and children. In a four
year study conducted in China, in which 36,000 children received
.5 to 1 mg sodium selenite per day, the incidence of Keshan
disease in the study group was 94% less than in the control group
(29). In Sweden, the lowest cardiovascular death rate is in the
city of Malmo, which has a high tap water selenium content (30).
In Mexico, patients given selenium-tocopherol capsules show a
decrease in incidence of angina (31). However, in New Zealand no
differences were found between blood selenium concentrations of
four groups of hypertensive patients and their healthy controls;
but selenium levels are low throughout New Zealand (31).
Selenium interacts strongly with numerous other elements. Its
toxicity can be alleviated by antimony, arsenic, copper,
germanium and tungsten, and it counters the toxicity of arsenic,
cadmium, mercury, silver and thallium (26). Its interaction with
magnesium is illustrated by the finding that cardiac-related
sudden death in transported pigs can be prevented by supplements
of either magnesium (32) or selenium (33). We consider the role
of selenium in cardiac function an unresolved question. Because
selenium has been so difficult to measure in drinking waters, it
has been impossible to carry out large-scale epidemiological
studies of this element.
TRACE ELEMENTS WHICH APPEAR TO PLAY A ROLE IN CARDIOVASCULAR
FUNCTION
Cadmium
Cadmium is an element toxic to man that is primarily an
inhibitor of respiratory processes. Cadmium levels in tissue,
particularly kidney cortex, increase with age, being almost nil
at birth. Higher levels of cadmium are found in highly
industrialized areas where the incidence of CVD is also high
(7,34,35).
Revis (14) has reviewed the in vitro and in vivo effects of
cadmium. In vitro, it decreases myocardial contractility, and
prolongs the PR interval and AV conduction time. In vivo, rats
exposed to cadmium develop prolonged PR intervals, and rats,
rabbits and pigeons develop hypertension. However, the
development of hypertension depends on the dosage: rats given
drinking water containing 5 ppm cadmium dropped their blood
pressure, while those fed water with 50 ppm cadmium sustained an
increase in blood pressure.
In humans, cadmium decreases serum cholesterol but increases
deposition of lipids in the walls of the aorta (36,37). A number
of studies have shown it to be present in higher concentrations
in the kidneys and urine of hypertensive patients as compared to
normotensive controls (38,39,40), although at least one author,
Morgan (41), failed to confirm these findings. Epidemiologically,
a study of 29 North American cities showed a positive correlation
between the levels of cadmium in the air and the incidence of
hypertension and arteriosclerosis (42). However, neither cadmium
workers nor Japanese with "itai-itai" (a cadmium-induced disease
giving rise to spontaneous fractures of bone) seem to be at
higher risk of developing hypertension (7,10).
Cadmium’s toxicity is potentiated by a low protein diet
and counteracted by zinc and selenium (43). Indeed, it may be the
cadmium-to-zinc ratio rather than the cadmium concentration which
is the determinant of hypertension.
Lead
Lead is a persistent bioaccumulative body poison. Its main
effects are hemolytic anemia, kidney disease and disturbances of
the central nervous system (44,45). Stofen (46), after reviewing
the effects of environmental lead on the heart, concluded that
lead may play a role in cardiovascular disease.
Electrocardiographic changes with prolonged PR intervals were
reported on rats perfused with lead (47). Lead has also been
reported to uncouple oxidative phosphorylation and inhibit the
NA+, K+-ATPase (48). Through this mechanism or perhaps by
interfering with the metabolism of cellular calcium, lead has
been reported to affect both the electrical and contractile
properties of the cardiovascular system (14). Lead has also been
shown to induce systolic hypertension in pigeons fed .8 ppm lead
for six months, but this effect is not seen in rats (14). Lead
toxicity is potentiated by low calcium intake and its absorption
from the gut is decreased by the presence of calcium. A recent
review by Saltman (49) suggests a role for lead in hypertension
because of its damage to renal tissue but the mechanism remains
obscure. Decreased levels of plasma renin and aldosterone have
been seen in lead poisoning. Increased levels of catecholamines
together with decreased responses of baroreceptors have also been
reported. Lead may also directly constrict arterioles and
increase blood volume. In humans, a lead-induced cardiomyopathy
has been described in “moonshine" drinkers (50).
Chromium
In its trivalent form, chromium is a well-known essential
element. Its major role is as a co-factor with insulin to
maintain normal glucose tolerance. Evidence of a cardiovascular
action came initially from Tipton (51) and Schroeder (52), who
showed a significant decrease in tissue levels of chromium with
age in populations having a high incidence of cardiovascular
disease. Trace amounts fed to rats prevented formation of
atheromatous lesions, decreased cholesterol levels and prolonged
their overall life-span, while its deficiency caused an increase
in the prevalence of aortic plaques (9,53,54). In humans,
however, the evidence for a role in cardiovascular function is
based on the well-known relationship between diabetes and
arteriosclerosis.
Copper
Copper is an essential element necessary for optimal
absorption and metabolism of iron, normal erythropoiesis and bone
collagen formation. Numerous authors report increased serum
copper levels in patients and animals with arteriosclerosis,
hypertension or myocardial infarction (55-60). Harman (61) has
also found that experimental animals who have higher intakes of
copper have more arteriosclerosis. He has postulated that
individuals prone to coronary artery disease may be identified by
a high serum copper level. Rats deficient in copper have abnormal
ECG’s, specifically ST changes and bundle-branch block
(62). Animals deficient in copper die suddenly and the pathology
shows myocardial degeneration, focal necrosis, fatty changes,
subendocardial fibroplasia, aneurisms and infarction (62).
It has been hypothesized that an imbalance in zinc and copper
metabolism may play a role in coronary heart disease (63). Copper
and zinc are biochemical antagonists and both contribute to the
control of the blood lipid levels. High zinc to copper ratio and
high risk of mortality or hypercholesterolemia have.been found
associated with diets high in fat or sucrose and in hypertension
(62). Klevay suggests that the mean copper intake described in
nutrition texts, of 2-5 mg/day, is out-of-date. He found a
geometric mean intake of .82 mg/day. Daily requirements are
1.55-2.1 mg/day (62).
Zinc
Zinc is essential for the life of all plants and animals. It
is both a co-factor and component of many metallo-enzymes and it
is present in all the tissues of the human body. Zinc deficiency
in humans seems to be associated with poor growth and
development, impaired wound healing, impairment to sensory
perception and, in all probability, with congenital abnormalities
(9,52).
A beneficial effect of zinc therapy has been reported in
atherosclerotic patients (64,65,66). It has also been reported
that zinc in rats reverses cadmium-induced hypertension. Several
investigators have found decreased zinc concentration in plasma
or serum after myocardial infarct (66). The levels return to
normal values after a few days (67). It is hypothesized that this
reflects a migration of the metal from plasma to tissues and that
it is a non-specific reaction to myocardial infarction (68).
However, Wester found that zinc concentrations decrease in
injured heart tissue after myocardial infarction. More recently
many of the effects of zinc have been attributed to the ratio of
zinc to copper (63).
OTHER ELEMENTS WHICH PLAY A ROLE IN CARDIOVASCULAR
FUNCTION
Calcium
Calcium is essential for man. It is a main structural element
necessary for blood clotting and for normal functioning of nerve
tissue. It is possible that certain types of cardiac disease are
aggravated by the lack of calcium, as it is required for muscle
contraction and it has been shown to decrease serum lipid levels.
Its role in cardiovascular function is however an indirect one.
Calcium prevents the absorption and transfer of toxins from the
intestine into the blood, and acts as a biological antagonist to
magnesium (69).
Sodium
The relationship between sodium and high blood pressure has
been confirmed by clinical and experimental studies during the
past 40 years. Clinical observations made as early as 1944 by
Kemper indicated that a low sodium diet was helpful to
hypertensive patients. Animal experimentation results were
reviewed by Calabrese and Tuthill (70) who indicated that: 1) the
greater ingestion of salt the more severe the hypertension, 2)
the younger the animal at the time it is first exposed to a high
salt diet the more it develops hypertension, 3) even a brief
exposure of two to six weeks to a high salt intake early in life
may influence the development of permanently elevated blood
pressure, 4) genetic factors influence the individual response to
salt. In animal studies as well as in human studies the
sodium:potassium ratio rather than sodium concentration alone has
been found to be related to hypertension. An association between
blood pressure and the amount of salt in the diet has been
observed in many different countries (71,72,73). It has, however,
been difficult to find a consistent correlation between salt
consumption and blood pressure within individuals in
industrialized countries. The best evidence to date comes from a
study by McGregor et al. (74) in which placebo and real salt
tablets were used in a double-blind intervention trial. Blood
pressure levels were consistently higher during the period in
which the patients were taking the real sodium tablets. Urinary
sodium levels confirmed increased sodium intake.
Magnesium
The role of magnesium in cardiovascular function will be
discussed later.
As far as the epidemiologists are concerned, our interest in
trace elements has been tied to what has become known as
‘the water story’.
Ever since Kobayashi (75), in 1957, noted a parallel between
the geographic distribution of the acidity of water in Japanese
rivers and the distribution of stroke, a major cause of mortality
in Japan, an increasing number of investigators all over the
world have attempted to elucidate and confirm the inverse
association between water hardness and mortality, particularly
mortality from cardiovascular causes.
As remarkable as the geographic diversity of these studies is
the great diversity of the hypotheses that have been favored by
different investigators, regarding both the identity of the
water-borne factor and the nature of the disease or pathologic
process induced by it. As well as mortality from cardiovascular
disease, mortality from all causes, chronic bronchitis, cancer,
and congenital malformations have been found to be associated
with soft water.
Numerous mechanisms have been postulated to account for the
association between minerals and cardiovascular disease (76). The
two most likely are:
1. Minerals may contribute to the incidence of disease by
increasing the risk of hypertension, i.e., a toxic effect
mediated via hypertension.
2. Minerals may act to lower the case fatality rate by
protecting people from sudden death.
The hypertension
theory. The elements involved in the hypertension theory
are cadmium, sodium and lead, thought to have toxic effects; and
calcium, thought to protect from the effects of these toxic
elements (77). Thus, there are several different hypotheses
within this theory, each implicating different elements or
combinations of them.
Cadmium
In 1965, Schroeder (78) hypothesized that the mode of action
of soft water was through an increased risk of hypertension due
to cadmium, and that cadmium would be leaching from pipes through
the corrosive action of soft water. Since then, support for this
hypothesis has come from a number of sources: 1) At the
experimental level, Schroeder’s findings in rats have been
replicated and extended to other animals (79). 2) At the clinical
level (autopsies) evidence of increased cadmium or cadmium-zinc
ratios in the kidneys of hypertensive patients have been
confirmed in most studies (80,81) (although some associate low
zinc concentrations more to renal damage than to essential
hypertension) (82).
The possibility of cadmium acting through water, how received
the strongest support from evidence that would implicate a toxic
effect of cadmium with a protective effect of hard water: In this
context, Perry (83) has shown that the hypertensive effect of
cadmium in rats was inhibited when hard water was used as the
vehicle for administration of the metal.
Lead
Lead has also been implicated in the hypertension theory.
Support for its involvement comes from Beevers et al. (84), who
examined the blood and tap-water lead levels of 135 pairs of
age-sex-matched hypertensive and normotensive subjects (Table
3).
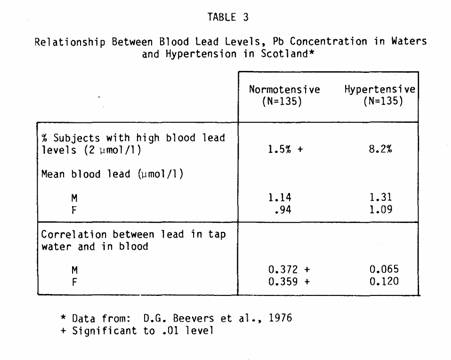
They found a significant excess of persons with high blood
lead levels among hypertensives; among normotensives they found a
positive correlation between blood lead and tap-water lead
concentrations. Sharrett (85), and Folsom and Prineas (86) have
reviewed the evidence implicating lead, and they conclude that if
the association between lead and high blood pressure exists, it
is probably only in areas with uncommonly high lead levels, such
as where lead piping and corrosive waters coexist.
Sodium
The hypertensive effects of sodium are well-established. What
remains unclear is what role sodium may play in the water story.
That salt in drinking waters may play a role is suggested from
the findings of Steinback et al. (87): when the village of
Juilovka was found to have one of the highest prevalences of
hypertension in the world — 45% — they searched for
an environmental factor and found that the water contained a
sodium concentration 26 times greater than in the nearby villages
in the Gurghiu Valley. However, it is more likely that dietary
sodium is the important factor in inducing hypertension, with
another waterborne factor having a protective effect. Crawford
(88) has suggested the ratio of magnesium and calcium to sodium
may be the important factor while Joossens (71) postulated that
calcium itself protects against sodium.
Calcium
The strongest epidemiologic support for the hypertension
theory as an explanation for the water story comes from
observations relating water hardness and blood pressure, again
suggesting that calcium may have a protective effect against some
of the toxic elements just discussed. Stitt (89), in the U.K.
reported on a sample of 244 civil servants living in 6 hard water
localities and a similar number living in 6 soft water
localities. The results of these observations are shown in Figure
2. It can be seen that not only are both systolic and diastolic
blood pressures higher in the soft water areas, but that this
difference becomes more marked in the older age groups. However,
Elwood (90) in Wales had previously found no significant
differences in mean blood pressure on similar-sized population
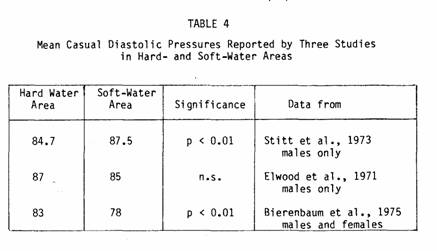
samples from hard and soft water areas (see Table
4).

Other types of studies also confirm an association between
water hardness (implicating calcium) and hypertensive mortality
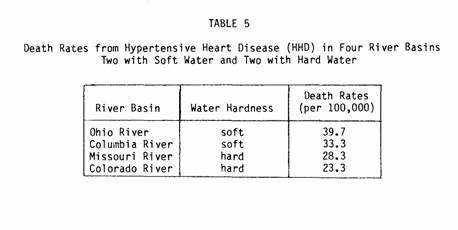
rates. For example, Masironi (91) reported a study comparing the
mortality of residents in four United States river basins, two
with hard and two with soft water. He found the death rates from
hypertensive heart disease to be 17% to 70% higher in the
soft-water basin (Table 5).
It must be remembered that our main interest is not whether
these elements can cause hypertension but whether they can
explain the water story. Thus, we must consider the intake of the
elements from water, relative to the intake from other sources,
keeping in mind factors such as chemical state and absorption. Of
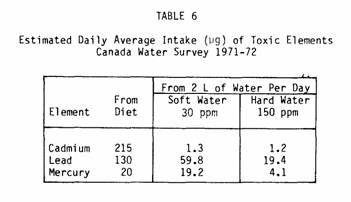
the three toxic elements discussed, cadmium is perhaps the
strongest candidate for a role in the water story, but cadmium
intake from drinking waters (Table 6) does not seem to be
substantial when compared, for example, to amounts absorbed from
cigarettes (92).
Hence, its role would have to be postulated in terms of
interaction with some other element, perhaps zinc, more abundant
in hard water than in soft water. Epidemiologically, the health
effects of two elements, one protective and the other toxic,
would be indistinguishable from the effects of the protective
element alone.
In summary, although there is good evidence that certain trace
elements and sodium may lead to hypertension, they do not seem to
adequately explain the water story. Therefore, in Canada we are
systematically pursuing the second theory — the idea of an
agent, present in hard-water areas, which is protective against
premature death, especially sudden death.
The sudden death
theory. In Canada we have conducted two studies in which
we progressively eliminated candidates from our original list of
elements and zeroed-in on those elements more likely to be the
water borne factor.
The first is a tissue study which provides for the comparison
of the metal content of the heart and of control muscles in
residents of hard- and soft-water areas, and specifically those
dying from myocardial infarction and from accidents (93). This
last group is taken as being representative of healthy subjects.
For the interpretation of this study, we established the
following criteria to assess the various elements implicated
(94):
1. The tissue concentration must differ between cardiac deaths
and accidental deaths.
2. The difference in tissue concentration must be consistent
with the postulated biological effect of a particular
element.
3. The difference in concentration among control patients from
each type of area must be consistent with the mineral content of
the water consumed.
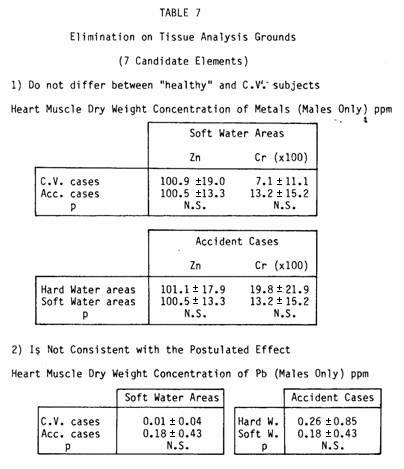
On this basis, we were able to eliminate zinc and chromium,
because the concentrations of these two elements are not
different in healthy and cardiac subjects (Table 7).
We also eliminated lead, because the results were not
consistent with its postulated toxicologic effect.
Similarly, we eliminated calcium and cadmium, which were found
in increasing concentrations in the hearts of subjects dying from
myocardial infarction as compared with accident cases, and
because the concentration of these elements does not differ among
healthy residents in soft- and hard-water areas (Table
8).
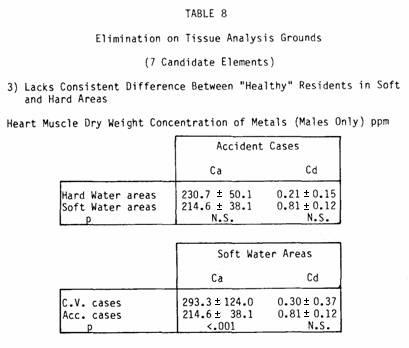
However, the increased concentrations of calcium in the
myocardium, while inconsistent with the hypothesis of a
protective effect of calcium, may substantiate findings such as
those of Van Barneveld (95) who noted sudden death among mice
receiving magnesium-poor, calcium-rich diets but no deaths among
mice receiving diets containing any other combinations of
magnesium and calcium.
We are now left with two possible elements, copper and
magnesium.
Copper levels are lower in the hearts of people who have had
heart attacks but they are also lower in healthy residents of
soft-water areas (Table 9).
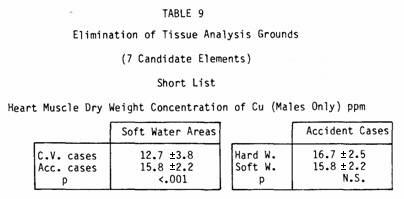
Here, there is a paradox, because the concentration of copper
in soft waters was about double that in hard waters, so one would
expect the tissue copper concentrations to be higher in soft
water areas.
Tissue magnesium levels showed the greatest difference between
the subjects dying from myocardial infarction and accident cases,
a difference that is present in both hard- and soft-water areas
(Table 10).
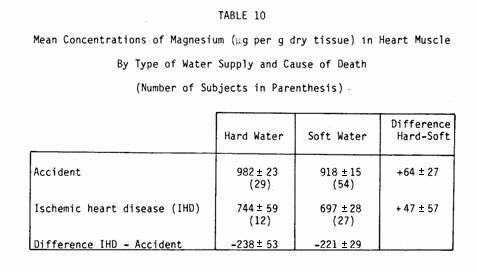
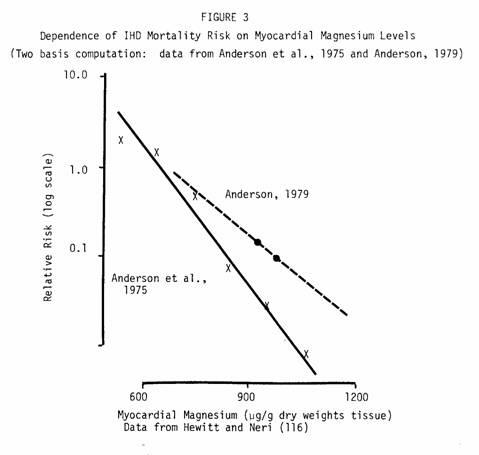
Moreover, the hearts of healthy residents in hard-water areas
also contained more magnesium than those in soft-water areas. The
data from this study can also be rearranged to portray variation
in individual risk as a function of individual myocardial
magnesium level (Fig. 3). Plotting the estimated relative risks
on a logarithmic scale and fitting them to a regression line
shows an impressive regularity of the data, despite small sample
sizes (only 122 subjects for all six datapoints). Even more
remarkable is the range of variation in apparent risk — by
a factor of approximately 200.
Thus, the tissue study implicates magnesium as the most likely
protective factor in hard water. The second study is an ecologic
study in which we examined the mineral content of drinking water
in relation to mortality (96). We randomly selected tapwaters
from more than 500 localities across Canada, each having a
communal water supply, and we measured 15 candidate elements,
chosen on the basis of suggestions by previous investigators
(97).
Again, we went through a sequence of progressive eliminations,
based on fixed criteria:
1. The suspected element must be found in the waters
consumed.
2. The trend seen with a particular element must be compatible
with the geographical distribution of hardness; that is, if the
element is protective, it must be present in larger quantities in
hard waters; if the element is toxic, it must be found in larger
amounts in soft waters.
3. A particular element must be present in sufficient
quantities in the waters consumed so as to make an appreciable
contribution to total dietary intake.
On this basis we could eliminate silver, selenium, molybdenum,
and antimony — all supposedly protective elements —
because they were detected in less than 10 percent of the
localities where waters were tested (Table 11).
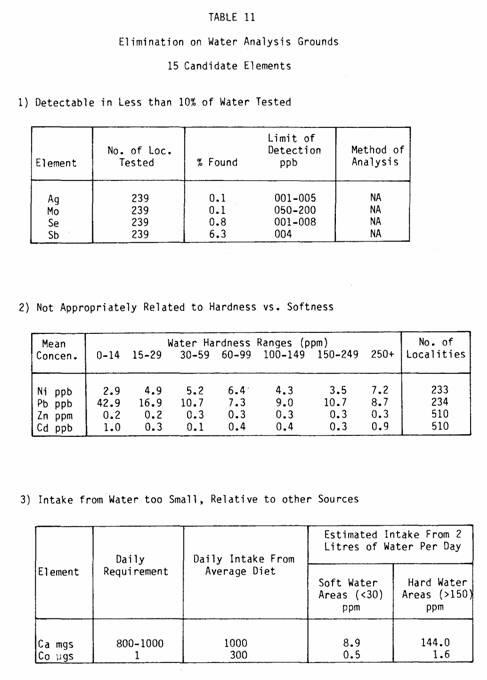
We also eliminated nickel, lead, zinc, and cadmium, because
these elements were not consistently related to the
hardness-softness gradient.
In addition we eliminated calcium and cobalt, because intake
of these elements from drinking water was too small in comparison
with intake from other sources.
Among the remaining five elements, there are three —
mercury, chromium, and copper — that can also be excluded
because of the lack of consistent supporting evidence from
studies in Canada and elsewhere (Table 12).
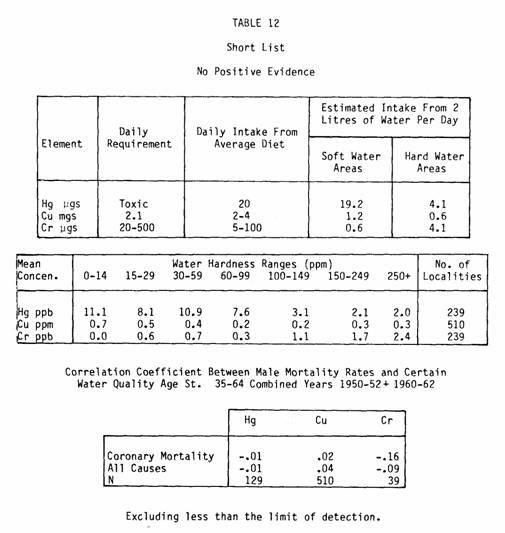
In fact, mercury, found in 60 percent of the sampled waters,
has a gradient of decreasing concentration with increasing
hardness. This could fit the hypothesis of a toxic element,
contained in soft waters, that provides an average of 10 µg
of mercury per day (i.e., five times the amount received from
hard waters). However, in the correlation studies, mercury does
not correlate significantly with mortality; in the few instances
in which it does, it has a negative sign indicating a protective
effect.
Chromium, found in 16 percent of the sampled waters, is found
in its highest values in hard waters. If the form of chromium
found in water is optimal for absorption, this could account for
a large proportion of the daily chromium intake in some
hard-water areas; however, mortality correlations in most
countries do not provide any conclusive evidence of a water-borne
chromium effect.
Copper also deserves more attention. It is found in decreasing
amounts with increasing hardness, and its highest values are
found in the softest groups of water. However, copper
concentration is not significantly associated with mortality.
This leaves magnesium and lithium (Table 13).
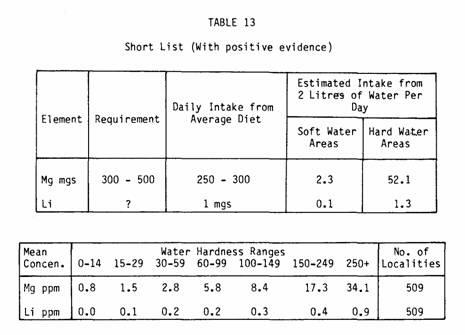
Magnesium is the strongest correlate in our correlation
matrix, that is, stronger than either calcium or hardness, with
lithium ranking fourth. Of course, it must be stated that all of
these factors are highly intercorrelated (Table 14).
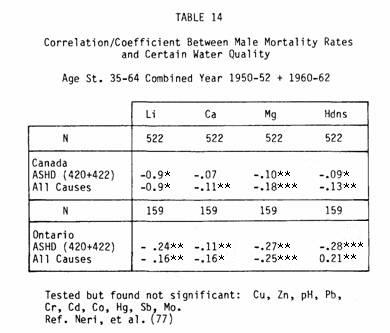
Thus our ecological study also implicated magnesium as the
most likely candidate for the water-borne factor. Confirmation
comes from other sources as well, the most recent being a report
by Leary et al. (98) in South Africa.
Does magnesium then satisfy all the criteria? What is its
dietary intake in relation to its requirements? The daily
requirement of magnesium is considered to be 300 mg for females,
350 mg for males and 450 mg for pregnant women. Average intakes
in various studies, ranges from 6-35% lower than the recommended
allowances, and for pregnant women may be as low as 57% of that
required (Table 15).
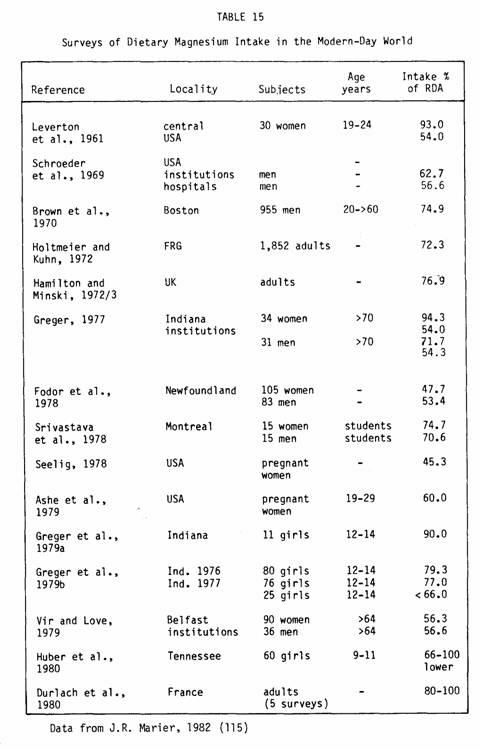
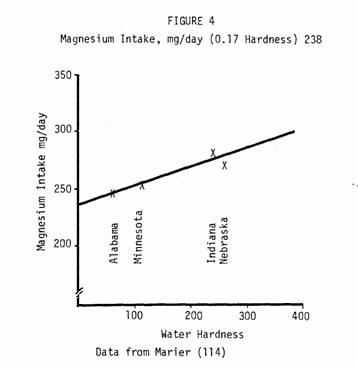
Thus the amount of magnesium ingested from water could make a
critical difference. Does it? Figure 4 shows magnesium intake as
a function of water hardness in four geographical areas of the
United States. The association appears striking, adding evidence
that sufficient magnesium can be ingested from hard water to make
the difference between inadequate and adequate intake. As well,
Binnerts et al. (99) have suggestive evidence that magnesium from
water is better and more rapidly absorbed than that from food.
Thus in terms of intake, magnesium seems to satisfy all the
necessary criteria.
What then, is the clinical and experimental evidence that
magnesium is likely to reduce cardiovascular mortality? The
literature in this field is voluminous and beyond the scope of
this paper but certainly supports the epidemiological evidence
(100). Some of the recent more important findings are that
magnesium supplementation in sub-pharmacologic doses stabilizes
arrhythmias (69), lowers blood pressure in hypertensives (101),
increases cardiac output (69) and reduces infarct size (102).
Subacute magnesium deficiency increases digitalis toxicity and
the multiplicity and severity of side effects from diuretics
(103) as well as causing changes in ECG patterns (104,105). At
the cellular level magnesium alters sodium transport (106) and
seems to safeguard against the influx of sodium and calcium into
cells (69). It also prevents catecholamine effects (107), being
protective against cardiotoxic substances and stress
(108-110).
Because of the long-recognized crucial importance of magnesium
as a vital cofactor for various enzymatic and metabolic
processes, a myocardium that is deficient in magnesium is likely
to be particularly vulnerable to a variety of stressful
situations. In fact, myocardial magnesium depletion can be
regarded as the "sensitizer" (111) in Hans Selye's (112)
"sensitizer-challenger" concept in which the "challenger" is most
probably a sudden surge of stimulus at the cardiac site.
Thus, epidemiological considerations, when viewed together
with the clinical and experimental work conducted in the area of
stress (we refer here to stress imposed on cardiac function),
suggest an hypothesis capable of tying together most of the
findings. This is the concept of the unstable, vulnerable heart,
which — possibly because of some metabolic deficiency
(likely related to magnesium deficiency) — is incapable of
responding to a sudden upsurge of functional demand. This can
conceivably happen in several situations in which the cause of
death would not be classified as cardiac on the death certificate
particularly in cases such as chronic bronchitis and maybe even
some of the cancers. The selective magnesium depletion found in
the myocardia of our tissue studies, in the study by Behr and
Burton (113) in England, and by various other studies in
experimental animals, is in line with this theory. One cannot
expect that the epidemiologic evidence collected up to now would
be able to confirm an hypothesis such as this. Studies will have
to be designed to test the specific hypothesis, and the ultimate
answer will hopefully emerge during a magnesium intervention
trial.
What then are our conclusions? If the myocardium can 1) become
selectively depleted in magnesium, and 2) can thereby become
impaired in its responses to a sudden demand for increased
cardiac output, we have the prerequisites for an increased
likelihood of fatalities, probably sudden fatalities, whether or
not they will be ultimately ascribed to the cardiac domain.
In terms of the Canadian experience, such an hypothesis is
fully applicable to the so-called "water story". It can also be
related to the inadequate intake of magnesium from dietary
sources, the so-called "empty-calories diet" of the modern-day
world.
REFERENCES
1 Z. Pisa and K. Vemura, World Health Organization Quarterly
Report, 35(1982) 12-21.
2 MRFIT Research Group, JAMA, 248(1982) 1465-1477.
3 I. Hjermann, I. Holmes, K.V. Byre, P. Leren, Lancet, 2(1981)
1301-1310.
4 J.T. Salonen et al., Br. Med. J., 2(1979) 1179-1183.
5 WHO European Collaborative Group. Europ. Heart I., 3(1982)
184-190.
6 L.C. Neri and D. Hewitt, Hardness of Drinking Water and
Public Health.
7 R. Masironi, Bull. WHO, 40(1969) 305-312.
8 Trace Elements in Human Nutrition. WHO Techn. Rep. Sev.,
(1973) 532.
9 J.C. Reinhold, Clinical Chem., 21(1975) 476.
10 R. Masironi, Europ. Colloquium on the Hardness of Drinking
Water and Public Health, Abstracts of Papers, Part 8, Luxembourg,
1975.
11 V. Ryabova, Vop. Biol. Med. Khim., Mater. Nauch. Brockhim.
Konf. 1st, 52(1968).
12 F.W. Sunderman et al., Geoch. Env. in Health and Disease,
N.Y. Acad. Sci., 199(1972) 300.
13 V.M. Sakharchuk et al., Kardiologiya, 12(1972) 131.
14 N.W. Revis, in E,W. Van Stee (Ed.) Cardiovascular
Toxicology, New York, 1982, pp. 365-375.
15 A.W. Voors; Am. J. Epid., 92(1970) 164.
16 H. Schroeder, Arch. Environ. Health, 28(1974) 303.
17 Y. Morin and P. Daniel, Am. J. Cardiol., 19(1967)
143-145.
18 C.S. Alexander, Am. J. Med., 53(1972) 395-417.
19 G.S. Wiberg, I.C. Munro and A.B. Morrison, Can. J.
Biochem., 45(1967) 128.
20 J.F. Van Vleet, A.H. Relon and V.J. Ferrans, Am. J. Vet.
Res., 38(1976) 991-1002.
21 A.W. Voors, Lancet, 2(1969) 1337-1339.
22 E.B. Dawson and W.J. Mcganity, 9th Int.. Congress of Nutr.
Abstracts, Mexico City 1972 p. 51.
23 M.L. Sievers and H.L. Cannon, Trace Subst. in Environ.
Health, 7(1973) 57.
24 R.A. Polumbo et al., Proc. Soc. Exp. Biol. Med., 142(1973)
1200.
25 P.A. Blachly, New Eng. J. Med., 281(1969) 682.
26 J.R. Marier and J.F. Jaworski, NRCC, No. 20643.
27 W. Hoekstra, in Hoekstra et al. (Eds.), Trace element
metabolism in animals, Baltimore, p. 61-77.
28 H. Vokal-Borek, Selenium USIP Report No. 79-16, Institute
of Physics, Univ. of Stockholm (1979).
29 R.J. Shamberger, Sci. Total Environ., 17(1981) 59-74.
30 H. Bostrom and P.O. Wester, Acta Med. Scand., 181(1967)
465.
31 C.D. Thomson and M.F. Robinson, Am. J. Clin. Nutr.,
33(1980) 303-323.
32 H.G. Classen et al., Arch. Pharmacol. (Suppl.), 307(1979)
41.
33 D.E. Ullrey, J. Anim. Sci., 51(1980) 645-651.
34 H.D. Livingston, Trace Subst. Environ. Health, 5(1971)
399.
35 H.M. Perry Jr. et al., Trace Subs. Environ. Health, 8(1974)
51.
36 H.A. Schroeder, J. Nutr., 86(1965) 51-66.
37 H.A. Schroeder and J.J. Balassa, Am. J. Physiol., 209(1965)
433-437.
38 J.N. Mackenzie and S. Kay, New Zealand Med. J., 78(1963)
68.
39 J. Iener and B. Bibr, Lancet, 1(1971) 970.
40 V. Karlicek et al., Cas Lek ces, 110(1971) 756.
41 J.M. Morgan, Arch. Int. Med., 123(1969) 403.
42 R.E. Carrol, JAMA, 198(1966) 267-269.
43 W.K. Tai, Water Hardness, Toxicity of Metals and Their
Relationship to Cardiovascular Disease. Division of Public Health
Engineering, Department of National Health and Welfare, Ottawa,
1970.
44 Biologic Effects of Atmospheric Pollutants — Lead:
Airborne Lead in Perspective, National Academy of Sciences,
Washington, D.C., 1972.
45 J.J. Chisolen Jr., Sci. Amer., 1971, 335.
46 D. Stofen, J. Mol. Cell Cardiol., 5(1974) 285-290.
47 S.J. Kopp et al., Toxicol. Appl. Pharmacol., 46(1978)
475-487.
48 B. Nechay and J.P. Saunders, J. Environ. Path. Toxicol.,
2(1978) 283-292.
49 D. Saltman, Annals Int. Med., 98(1983).
50 S.K. Asokan, M.J. Frank and A.C. Wilham, Am. Heart J.,
84(1972) 13-18.
51 I.H. Tipton et al., Health Phys., 2(1965) 40-451.
52 H. Schroeder, Circulation, 35(1967) 570-582.
53 H.W. Staub et al., Science, 166(1969).
54 Schroeder et al., J. Chronic Dis., 23(1970) 123-142.
55 S.S. Adelstein et al., NEJM, 255(1956) 105-109.
56 A. Hanson and G. Biorch, Acta. Med. Scand., 157(1957)
493-502.
57 E.L. Kanabrocki et al., J. Nucl. Med., 8(1967) 166-172.
58 V.A. Kondurtsev, Ter. Arkh., 41(1968) 62.
59 I.D. Rachinskii, Kardiologya, 9(1969) 84.
60 A.V. Aronov, Kardiologya, 13(1973) 43.
61 D. Harman, Circulation, 38, Supp. 6 (1968) 8.
62 L. Klevay, J. Environ. Path. and Toxicol., 4-2, 3(1980)
281-287.
63 L. Klevay, Amer. J. Clin. Nutr., 28(1975) 764-774.
64 J.H. Henzel et al., Trace Subst. Environ. Health, 4(1971)
336.
65 J.P. Isaacs et al., Trace Subst. Environ. Health, 5(1972)
313.
66 L.D. McBearn, Clin. Chem. Acta., 50(1974) 43-51.
67 A.M. Handjani et al., Chest, 65(1974) 185-187.
68 P.O. Wester, Acta. Med. Scand., 178(1965) 765-788.
69 O. Lehr, Magnesium Bull., 3(1981) 178-191.
70 E.J. Calabrese and R.W. Tuthill, Arch. Environ. Health,
32(1977) 200-202.
71 J.V. Joossens, Triangle (Engl. Ed.) 12(1973) 9-16.
72 A.G. Shaper et al., Afr. Med. J., 46(1969) 282-286.
73 N. Sakaki, Geriatrics, 19(1964), 735-744.
74 G. MacGregor et al., Lancet, 2(1982), 351-355.
75 J. Kobayashi, Ber. Ohara. Inst. Landwirtsch. Biol. Okayama
Univ., 11(1957) 12-21.
76 L.C. Neri, D. Hewitt, G.B. Schreiber, Am. J. Epidemiol.,
99(1974) 75-88.
77 L.C. Neri, H.L. Johansen, N.Y. Ann, Acad. of Sciences,
304(1978) 203-219.
78 H. Schroeder, J. Chronic Dis., 18(1965) 647-656.
79 G.S. Thind, J. Air Pollut. Control Assoc., 22(1972)
267-270.
80 H.M. Perry, J. Am. Dietetic Assoc., 62(1973) 631-637.
81 J.B. Lener, Lancet, 1(1971) 970.
82 G.S. Thind and G.M. Fischer, Clin. Sci. Mol. Med., 46(1974)
137-141.
83 H.M. Perry et al., 8th Annual Conference on Trace
Substances in Environ. Health, Columbia, Mo. 1974.
84 O.G. Beevers et al., Lancet, 2(1976) 1-3.
85 A.R. Sharrett, Amer. J. Epid., 110(1979) 401-419.
86 A.R. Folsom and R.J. Princes, Amer. J. Epid., 115(1982)
818-832.
87 M. Steinbach et al., Rev. Roum. Med., 13(1975) 261-263.
88 M.D. Crawford, Proc. Nutr. Soc., 31(1972) 347-353.
89 F.W. Stitt et al., Lancet, 1(1973) 122-126.
90 P.C. Elwood et al., Br. Med. J., 2(1971) 362-363.
91 R. Masironi, Bull. WHO, 43(1970) 687-691.
92 G.P. Lewis et al., J. Chronic Dis., 25(1972) 717-726.
93 T.W. Anderson et al., Can. Med. Assoc. J., 113(1975)
199-203.
94 L.C. Neri, J. Am. Water Works Assoc., 67(1975) 403-409.
95 A.A. Van Barneveld, C.J.A. Van den Hamer and J.P.W.
Houtman, Trace Subst. Environ. Health, 16(1982) 196-204.
96 L.C. Neri, J.S. Mandel, D. Hewitt, Lancet, 1(1972)
931-934.
97 L.C. Neri, H.L. Johansen, F.D.F. Talbot, Chemical Content
of Canadian Drinking Water Related to Cardiovascular Health,
Ottawa, 1977, p. 223.
98 W.P. Leary and A.J. Reyes, SA Med. J., 64(1983)
697-698.
99 W.T. Binnerts et al., In Trace Element — Analytical
Chemistry in Medicine and Biology, Vol. 2, Berlin 1983,
87-93.
100 J.R. Marier, L.C. Neri, T.W. Anderson, Water Hardness,
Human Health and the Importance of Magnesium, NRCC No. 17581,
1979.
101 T. Dyckner, O.P. Wester, Br. Med. J., 286(1983)
1867-1849.
102 B. Morton, Magnesium Bull., 3(1981) 192-194.
103 L. Cohen and R. Kitzis, JAMA, 251(1984) 730.
104 K.S. Drasner et al., Can. Anesth. Soc. J., 28(1981)
329-333.
105 C. Wan-Chun et al., Am. Heart J., 104(1982) 1115-1116.
106 P. Fisher and A. Giroux, Nutr. Res., 4(1984) 51-57.
107 A. Ebel and T. Gunther, J. Clin. Chem. Clin. Biochem.,
21(1983) 249-265.
108 B. Malviel-Shapiro, S. Afr. Med. J., 32(1958)
1211-1214.
109 R.S. Parsons et al., Med. Proc., (1960) 479-481.
110 A. Thurnherr and J. Kock, Med. Wochens Chr., 92(1962)
949-956.
111 L.C. Neri, J.R. Marier, H. Nato. (Ed.) Nutrition and Heart
Disease, N.Y. 1982, 81-96.
112 H. Selye, Anaphylactoid Edema, St. Louis Missouri WH Green
Inc., 1968.
113 G. Behr and P. Burton, Lancet, 2(1973) 450.
114 J.R. Marier, Rev. Canad. Biol., 37(1978) 115-125.
115 J.R. Marier, Magnesium, 1(1982) 3-15.
116 D. Hewitt, L.C. Neri, J. Environ. Path. Toxicol., 4(1980),
51-63.
This page was first uploaded to The Magnesium Web Site on
September 18, 2002
http://www.mgwater.com/



















