Magnesium Research (1995) 8, 1, 65-76
Review paper
Red blood cell magnesium concentrations: analytical problems
and significance
H. Millart*, V. Durlach† and J. Durlach‡
*Laboratoire de Pharmacologie-Toxicologie, Hôpital
Maison Blanche, 51092 Reims Cédex, France;
†Clinique Médicale U62, Hôpital Robert Debre,
51092 Reims Cédex, France; ‡Président de la
SDRM (Hôpital St. Vincent-de-Paul, Paris), 64 rue de
Longchamp, 92200 Neuilly/Seine, France
Summary: In order to assess total magnesium
concentrations in human red blood cells (erythrocytes--ErMg),
atomic absorption spectrometry (AAS) provides high accuracy and
precise method rapid and amenable to automation. Taking care of
eliminating the chronic marginal magnesium deficits, normal
values of ErMg evaluated through a direct method and expressed
as mmol/litre of packed cells are 2.3 ± 0.24.
Inductively coupled plasma-mass spectrometry (ICP-MS) is a
multielemental analytical technique. Particle induced x-ray
emission (PIXE) also provides multielemental capability, but is
time-consuming and costly. Microelectrodes are the gold
standard for intracellular Mg2+ measurements. But
microelectrodes and fluorescence probes measure the activity of
magnesium ions, not the concentrations. Ionized magnesium
content of human intact erythrocyte is mainly assessed with the
NMR method and with the zero point titration. The concentration
of ionized magnesium as estimated by NMR (31P NMR method) was
found to be 0.20 ± 0.02 mmol/litre cell water and with
the zero point titration 0.55 ± 0.12. The uncertainty
concerning the two current used techniques for free magnesium
determination is worsened by the fact that magnesium inside red
cells continually oscillates in vivo. Free magnesium
constitutes a small part of total magnesium. Further studies
are necessary to assess the importance of its variations in
clinical medicine. Efflux of ErMg is controlled through
membranous sodium-dependent and sodium-independent pathways and
through genetic and neurohormonal regulations. Variations in
the total or ionized ErMg do not necessarily mean that similar
changes should exist in the magnesium pool. But it remains the
basic static cellular magnesium item. Its value will be
subsequently enhanced when it takes place among the clinical
and paraclinical data of dynamic magnesium investigations.
Key words: Atomic absorption spectrometry
(AAS), erythrocyte, fluorescence, genetic and neurohormonal
control, ion selective electrode (ISE), magnesium, nuclear
magnetic resonance (NMR), null point, particle-induced X-ray
emission (PIXE), red blood cell (RBC).
Introduction
During acute or chronic experimental magnesium deficiency a
decrease in erythrocyte magnesium (ErMg) is observed. It occurs
more slowly and discreetly than in plasma magnesium. The
measurement of this cellular magnesium item being easy, it has
been conferred a primary role in routine static analysis of total
or ionized cellular magnesium. Concentration of erythrocyte
magnesium is related to their age and their quick renewal goes
with an increase of erythrocyte magnesium unrelated to a
magnesium overload.
The dogma of the lack of exchange between erythrocyte and
plasma magnesium is far from being intangible. Red blood cells
are distinct from the other cells of the organism as they have
numerous specific characteristics namely the absence of nucleus
and of mitochondria and therefore have a particularly low
cellular magnesium concentration. But it remains none the less
true that in clinical practice, erythrocyte magnesium
concentration constitutes the easiest way of investigating
cellular magnesium.
The aim of this mini-review is:
(1) To evaluate the current analytical techniques for measuring
total magnesium and free magnesium in red blood cells;
(2) To sum up our present knowledge on membranous and systemic
control of the red cell magnesium content.
And to conclude on the interest of erythrocyte magnesium
evaluation as the best routine static cellular magnesium item for
the investigation of magnesium status. Its value will
subsequently be enhanced when it takes place among the clinical
and paraclinical data of dynamic magnesium investigations.
The determination of total magnesium
Atomic absorption spectrometry of magnesium
Instrument parameters
The most widely used technique for measuring total magnesium
is atomic absorption spectrophotometry (AAS)1. The
sensitivity, defined as the concentration required for 1 per cent
absorbance, is about 0.01 µ /ml for flame AAS at the 285.2
nm resonance line, while electrothermal (graphite furnace)
AAS(ET-AAS) has a sensitivity of 0.17 pg2. The
sensitivity of magnesium measurement by flame atomic absorption
is sufficiently high that the graphite furnace instrument is
infrequently required for biological determinations. The flame
most often used is air-acetylene since it is sensitive, prohibits
many interferences, and is readily available. While a fuel-rich
air-acetylene flame has a greater sensitivity towards magnesium,
an oxidizing (fuel-lean) flame is less susceptible to some
interferences3. Thus, as a general rule, an oxidizing
flame is recommended except when extreme sensitivity is
required.
Interferences
The worst interferences in magnesium AAS are observed with
metals that form acid oxides that are stable at high
temperature4. These elements, which include aluminium,
silicon, titanium and zirconium, cause severe interferences only
when present in relatively high concentrations4. Many
other metals have been reported to interfere with magnesium AAS
under various conditions including lithium, sodium, potassium,
rubidium, chromium, selenium, beryllium, iron, vanadium,
molybdenum, caesium, strontium, calcium, and
barium,5,6. Chloride, oxalate,
ethylenediaminetetraacetic acid (EDTA), and 8-hydroxyquinoline
have been reported as anion interferences5,6. Although
a large number of interferences exist, most are easily overcome.
An air-acetylene flame prevents interferences due to sodium,
potassium, calcium, phosphate and iron7. The presence
of 0.1-1 per cent (w/v) lanthanum chloride or strontium chloride
eliminates the remaining interferences except for those caused by
chromium and titanium50. EDTA (0.4 per cent) overcomes
the interference of chromium. Some commonly used biological
buffers cause little or no interference of magnesium AAS. High
(non-physiological) concentrations of phosphate have been
reported to interfere5,7. However this interference is
not seen with either an air-acetylene flame or a strontium or
lanthanum diluent5,7. Metal-free solution of 50 mM
Tris, Mes, or HEPES do not interfere with the determination of
0.25 µ/ml magnesium1. Protein has occasionally
been reported to interfere8,9. In these cases, this
effect is probably due to high sample viscosity which may alter
the sample aspiration rate. Dilution of the sample is generally a
convenient remedy to this problem. Factors that exacerbate
interferences are a cool flame and the presence of nitrate or
sulphate4,6.
Means of assessing magnesium concentrations in human
erythrocytes
Direct method: The assay of
intracellular magnesium concentration in erythrocytes is
performed in fresh erythrocytes which are washed three times with
ice-cold solutions such as 140 mM choline chloride, 100 mM
MgCl2, a Tris buffer pH 7.40 containing 146 mM choline
chloride, 1mM MgCl2, 1 mM CaCl2, 5 mM
ortho-phosphoric acid, a solution containing 75 mM
MgCl2, 85 mM sucrose and 10 mM Tris-MOPS pH 7.40, a
sodium phosphate-saline buffer pH 7.4010, or with 0.15
M saline 11.
After cell washing the recentrifugation of the cell suspension
at higher g forces and longer times than 1200 g
for 5 min does not result in further packing of the
erythrocytes12.
Fluorescence polarization studies revealed a 15 per cent
increase in the fluidity of membranes from magnesium-deficient
rat erythrocytes and analysis of the membranes showed decreased
amounts of magnesium13. Thus severe magnesium
deficiency might change optimum centrifugation conditions.
The cells are then diluted and lysed with ice-cold bidistilled
water. To speed up haemolysis, 0.2 per cent purified saponin is
sometimes used 14.
After cell lysis the membranes are removed by centrifugation
at 2000 g for 20 min. The haemolysate is diluted with an
acidic solution, for example 0.1 M HCL)4 + 125 mM S r2
+10 or 0.3 M HCL containing 0.5 per cent (w/v) lanthanum
chloride11 and the analysis is performed in an atomic
absorption spectrophotometer. Usually erythrocyte magnesium
concentrations are expressed as mmol/litre of packed cells (mean
± SEM) (2.08 ± 0.04), sometimes as mmol/kg wet
weight (1.33 ± 0.06), as mmol/kg dry weight (4.03 ±
0.17)10, or as ng/10E6 cells (3.63-6.42) and
µ/grams of haemoglobin15. But these red blood
cell magnesium concentrations have been established without
taking care of eliminating the marginal chronic magnesium
deficit. If that is done, normal erythrocyte magnesium
concentrations expressed as mmol/litre of packed cells are 2.30
± 0.2414,40.
Indirect method: Although the direct
method is considered to be more nearly accurate, some have
speculated that part of the observed variability may be due to
the distribution of cells in the erythrocyte pellet. Nucleated
erythrocytes and reticulocytes, cells known to have higher
concentrations of magnesium than mature erythrocytes, would be
distributed at the top of the pellet. Thus the magnesium content
of a specimen could be affected by the cell-size distribution of
the final aliquot used for analysis.
Deuster et al. evaluated three methods (two indirect
and one direct) for determining the magnesium content of red
blood cells, to compare methodological differences and to
establish a method suitable for use in field studies. For the
indirect methods, erythrocytes in whole blood were lysed by
adding either de-ionized water (I) or nitric acid, 2 mol/litre
(II). For the direct method (III), erythrocytes were isolated by
density centrifugation, washed, then digested in concentrated
HNO3. Magnesium concentrations were measured by atomic
absorption spectrophotometry in plasma and whole blood for the
indirect method, and in the pellet for the direct method.
Haematocrit and haemoglobin were measured, and erythrocytes were
sized and counted on all samples. When values for the three
methods were compared, that by method I was significantly lower
than those by methods II and III. Values obtained by method II
were 100.1 percent of that by the direct method. The indirect
method with 2 mol/litre HNO3 lysing solution provides
a reproducible, reliable, accurate, and simple technique for
measuring magnesium in erythrocytes (micrograms per gram of
haemoglobin): results (method II) in micrograms/gram of
haemoglobin were (mean ± SD) 116.6 ± 14.7.
Other techniques
Intra-erythrocytic magnesium assay in the Kodak Ektachem 700
analyser16
Bonnay et al.16 used a Kodak Ektachem 700
to measure red blood cell magnesium by using the neutralized
supernatant of the perchloric acid-treated haemolysate. The
supernate is used as if it were a plasma sample (overall dilution
1:6) in the Ektachem.
Particle-induced x-ray emission (PIXE)17
X-ray emission can be induced by particle beams (PIXE). During
particle excitation of material, characteristic x-rays are
emitted from target atoms. The PIXE method, used in conjunction
with these microbeams, provides unique possibilities due to a
combination of good analytical sensitivity, good spatial
resolution and multielemental capability. The beam is reduced to
microscopic size by an original designed microbeam line. The beam
section can be adjusted from 1.5 µm to 10µm.
Micro-PIXE is one of the few techniques able to quantify and
locate an element within a cell. Micro-PIXE has enough qualities
to become a powerful routine method in biology but it is still
time-consuming and needs a particle accelerator which is only
available in nuclear centres. The competitive methods SIMS
(secondary ion mass spectrometry) and SEM (scanning electron
microprobe) are either monoelemental or less sensitive but
achieve best spatial resolutions.
Various techniques have been used to obtain erythrocyte
populations of various ages. Most of the investigations of
erythrocyte aging have been based on the assumption of an
age-dependent difference in erythrocyte density. Given that the
potassium concentration (mM/kg dry mass) of an individual
erythrocyte can be used to mark the degree of senescence of the
erythrocyte and given that electron-probe x-ray microanalysis
technique allows the quantitative analysis of multiple elements
in each erythrocyte then it becomes feasible to study systematic
variations in the ionic concentration of the erythrocyte during
the senescence process. Such a study circumvents the need for
erythrocyte separation procedures. Specifically as potassium and
Cl decreased in concentration, calcium increased in concentration
whereas sodium and magnesium did not demonstrate a significant
pattern of change. These findings are in keeping with the past
observations that erythrocyte senescence is accompanied by:
decreased water, decreased potassium and decreased volume, in
addition to increased density and increased
calcium12.
Accurate measurement of stable isotopes of magnesium in
biological materials with inductively coupled plasma mass
spectrometry (ICP-MS)
Schuette et al.18 recently described a
general method for the accurate isotopic determination of
magnesium (24Mg25Mg26Mg) in
biological materials, which is based on inductively coupled
plasma mass spectrometry (ICP-MS).
Conclusion
By itself, AAS is an analytical technique capable of providing
high accuracy and precision of trace element quantitation.
Generally, flame AAS is quite selective, rapid, amenable to
automation, and therefore the technique of choice whenever
adequately sensitive. Background absorption is not a particular
problem and is completely compensated for by using a deuterium
background corrector; however, it should always be checked for.
Transport interferences are encountered with viscous sample
solutions, and may call for viscosity-matched standards. inasmuch
as high-salt solutions are often inevitable in flame AAS, it is
essential to thoroughly optimize observation height and acetylene
flow, so as to avoid time-consuming standard addition
calibrations. Generally recognized assets of electrothermal AAS
(ET-AAS) are its low detection limits, small size sample
requirements, and the possibility of direct sampling. As such it
is a method of choice. It has, however, some drawbacks and
limitations: serious background problems, severe interferences,
carbide formation, more complicated calibration, more critical
optical alignment, problems with measuring fast transient
signals, pipetting errors, pronounced sensitivity drift and
memory effects, higher contamination risk, much longer
instrumental time (e.g., 2-4 min per sample vs.
5-10 s in flame AAS), higher qualification of operator required,
and higher cost.
Inductively coupled plasma-mass spectrometry (ICP-MS) is a
multielement analytical technique. Its analytical domain overlaps
both those of ICP emission and graphite furnace atomic
absorption. For example, atomic spectroscopy detection limits
(micrograms/litre) are 0.1 for flame AAS, 0.004 for ET-AAS, 0.08
for ICP emission and 0.007 for ICP-MS. In short, ICP-MS offers
the analytical productivity of ICP emission with the detection
limits of ET-AAS. ICP-MS is also being used for stable isotope
tracer measurements. In this technique, chemicals enriched in one
or more stable isotopes of an element are added to a dynamic
system to trace the flow of the added element through it. Typical
application is in the biomedical field for dietary studies. The
enriched stable isotopes are relatively easy to obtain and are
easier to work with than radioisotopes for tracer work.
Micro-PIXE is one of the few techniques able to quantify and
locate an element within a cell. Besides spatial resolution
Micro-PIXE provides multielemental capability. However this
technique is time-consuming and costly.
Free and bound cytoplasmic magnesium in red blood cells
Introduction
While magnesium ions can modify numerous cell processes in
vitro, their in vivo role remains unclear. The main
reason for the uncertainty regarding the intracellular role of
magnesium was, until recently, the technical difficulties of
measuring the free or ionized concentration of the ion, for it is
the free and not the total concentration that is the key
physiological parameter 19.
Magnesium can bind to proteins or anions. Due to the binding,
an equilibrium is established between the free magnesium and the
bound magnesium. If the concentration of the total number of
binding sites is (X)T and the total magnesium is
(Mg)T, and K the equilibrium constant, free magnesium
concentration (Mg2+) is given by the following
equation19:
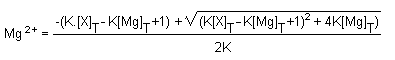
Thus the concentration of the free magnesium concentration in
cells depends on the equilibrium constant K, as well as on the
total concentrations of either magnesium or binding sites. The
methods that have been used in the past to measure the ionized
magnesium is measured directly with microelectrodes and should
thus give the most accurate estimations of the free magnesium
levels in the tissues. In the second group, the ionized magnesium
is not directly determined, but is estimated from some reaction,
depending on the ionized magnesium concentration.
Previously, the concentration of ionized magnesium in red
cells was estimated using adenylate kinase equilibrium in intact
cells and red cell lysates, since this enzyme requires magnesium
as an activator20.
Then the molal concentration of ionized red blood cell
magnesium has been measured with a magnesium electrode in a
stroma-free, freeze-thaw haemolysate of fresh human oxygenated
red blood cells with a divalent cation-specific electrode; a
value of about 0.5 mM was found21.
From the numerous approaches three are now routinely used to
study the intracellular free magnesium concentration and its
regulation, namely: magnesium sensitive microelectrodes,
fluorescence probes (Mag-Fura) and nuclear magnetic resonance
spectroscopy (NMR).
The microelectrodes are the gold standard for intracellular
magnesium measurements
Magnesium ion activities are evaluated by ion-selective
electrodes. They are usually transformed into ion concentrations
using a calibration procedure. This transformation, which is
ultimately based on the calculation of activity coefficients, is
assuming a constant ion background (constant ionic strength, I).
Nevertheless it is the activity coefficient of the calibration
solution which is strictly constant. This is not the case even
with a biological sample. It would be more adequate to specify
activities. Matrix interaction, degree of discrimination of
background ions (selectivity), life time of the membrane, and
dynamic response behaviour have to be taken into account to avoid
systematic errors. Actually the ionophore ETH 7025 (N', N'',
N'''-imino-di8, 1-octanediyl) tris
(N-heptyl-N-methyl-malonamide) is the only one
with a pronounced discrimination of calcium ions and a
sufficiently high selectivity to monovalent ions, suitable for
measurements in blood22.
Fluorescence probes for free magnesium assay
In the absence of divalent cations, the fluorescence
excitation spectrum of furaptra shows a maximum at 370 nm when
the emission wavelength is set at 510 nm. Upon addition of
magnesium or calcium, the fluorescence excitation maximum of
furaptra is 335 nm23. In contrast, addition of
magnesium or calcium causes a decrease in intensity but not a
shift in the wavelength of the fluorescence emission maximum at
510 nm with the excitation wavelength set at 370 nm. Because of
the shift in the excitation maximum upon magnesium binding, the
free intracellular magnesium ((Mg2+)f) concentration
measured from the fluorescence excitation spectrum of furaptra is
obtained from the ratio method according to:
(Mg2+)f = KD Smin
(R-Rmin)/maxXR(max-R) where R is
the fluorescence intensity ratio at the wavelengths of 335 and
370 nm observed with the biological sample, Rmin and
Rmax are the fluorescence intensity ratios in the
absence and presence of saturating amounts of magnesium and
Smin and Smax are the fluorescence
intensities in the absence of magnesium and in the presence of
saturating magnesium, respectively. The main advantage of the
ratio method is that magnesium measurements are independent of
the concentration of the fluorescence indicator used. The
fluorescence intensity measured for the sample must be corrected
for cell autofluorescence by subtraction of the measurements
observed with unloaded cells at the wavelengths of 335 and 370
nm. The fluorescence emission spectrum is the mirror image of the
absorption spectrum.
The normal cytosolic free calcium concentration in most cells
is about 100-200 nM, a value 250-500 times lower than the
apparent KD for calcium binding to furaptra. Thus under basal
conditions, only approximately 0.2-0.4 per cent of the furaptra
will be complexed with calcium whereas approximately 33-36 per
cent of the furaptra will be magnesium complexed since basal
Mgi is 0.5-1 mM. Furaptra in solutions which mimic the
intracellular milieu has an apparent KD-Mg of 1.5 mM, an apparent
KD-Ca in the range of 20-60 µM, and a PK of 5.0. Furaptra
is now available from Molecular Probes (Eugene, OR, USA) under
the designation Magfura-2. In spite of the limitations mentioned
above, furaptra has been extensively used to measure
Mgi in numerous cells: BC3HI cells, 3T3 fibroblasts,
peripheral blood lymphocytes, MDCK cells, pancreatic
β-cells, vascular smooth muscle line A7r5, isolated
myocytes, kidney cells (MDCK), hepatocytes24.
Null point for plasma membrane permeabilization using the
ionophore A23187
An alternative approach is provided by treating the cells with
the ionophore A23187 and Mg 2+25. Using a divalent
cation ionophore A23187 the authors have investigated magnesium
buffering in intact human red blood cells and have found that the
fresh, oxygenated, inosine-fed cell has three main buffer systems
which bind nearly 90 per cent of the total magnesium present
inside the cell in physiological conditions. Net magnesium
movements across the intact red cell membrane renders the cell
highly permeable to magnesium, allowing rapid equilibration of
magnesium ions across the membrane and with the intracellular
buffers. At electrochemical equilibrium the distribution of
magnesium ions is given by Mgi2, =
r2 Mgo2+, where
Mgi2+ and Mgo2+ are
the molal concentrations of ionized magnesium in cell water and
in the medium, respectively and r2 is the Donnan
distribution ratio for divalent cations at equilibrium. The total
magnesium content (Mgt) of the cells is the sum of the free and
bound forms (Mgb) where Mgt and Mgb are the molal concentrations
of total and bound magnesium in cell water. From these equations
bound magnesium is equal to Mgb = Mgt - r2
Mgo2, . The total magnesium content of the
cells was measured by AAS in the trichloroacetic supernatant of
cell lysates. Cell water was measured as the difference between
wet and dry weights of packed-cell sample corrected for
extracellular fluid. The original magnesium content of the cells
used in the present experiments was 3.46 ± 0.05 mmol/kg
cell water. The method described here can be used to investigate
the effect of metabolism and deoxygenation on the magnesium
buffer curve in intact cells as well as the magnesium dependence
of active sodium and calcium transport. The main disadvantages of
the null-point method are the following: (i) it does not give a
direct measurement of (Mg2+)i, (ii) fairly
dense suspensions of cells are required, and (iii) an independent
assessment of intracellular pH or, when appropriate,
r2 is also required.
The concentration of ionized magnesium in the oxygenated cells
was found to be 0.39 mM and was not greatly affected by changes
in the composition of the medium. The concentration of ionized
magnesium in deoxygenated cells showed more dependence on the
composition of the medium. Values of 0.54 and 0.62 mM were found
in cells incubated in TRIS- and HCO3 buffered media
respectively. Only a small increase of 0.16-0.22 mM was found in
the concentration of ionized magnesium when the cells were
deoxygenated. This increase might be due to a change in the
binding of the important magnesium chelators,
2,3-diphosphoglycerate (2,3-DPG) and ATP by
haemoglobin26.
Nuclear magnetic resonance (NMR) spectroscopy
Nuclear magnetic resonance (NMR) is a spectroscopic technique
that can be used to measure (Mg2+)i in
intact cells. The main advantage of NMR is that it is noninvasive
and therefore ionic and metabolic changes in the intracellular
environment can be monitored within an essentially unperturbed
living system.
31P nuclear magnetic resonance27
The measurement of (Mg2+)i by NMR is
based on the fact that the frequency separation between the
α and β or β and γ-phosphates of ATP depend
on the ratio of MgATP to unbound ATP. Therefore, the NMR spectrum
of intracellular ATP may be used to measure the fraction of ATP
that is completed to Mg2+ and, thereby, to estimate
the level of (Mg2+)i in intact cells. The
main disadvantage of the technique is that (Mg2+), is
not measured directly but through its effects on the NMR spectra
of 31P and the accuracy of the estimate of (Mg2+)
crucially depends on precise knowledge of the dissociation
constant for MgATP under physiological conditions
27.
The phosphorus NMR spectra of intracellular ATP in glycolysing
human red blood cells maintained at 37° C in an atmosphere
containing 5 per cent CO2 have been obtained and quantitated
under completely aerobic and anaerobic conditions. The results
showed that 84 ± 4 per cent and 78 ± 4 per cent of
the total ATP are completed to magnesium in the aerobic, and
anaerobic states of the cell, respectively. The intracellular
concentration of free magnesium was determined to be 0.25
± 0.07 mM in the aerobic and 0.67 ± 0.15 mM in the
anaerobic state in a sample of normal erythrocytes. The magnesium
concentrations calculated on a water basis were determined to be
1.02 vs 0.83 mM for ATPMg, 0.73 vs 0.80 mM for
ATPMgHb, 0.44 vs 0.20 mM for glycerate-2,3-P2 9DPG) Mg
complex (DPGMg), in aerobic and anaerobic conditions
respectively. Since the magnesium in the red cell is largely
complexed, the three-fold increase in free magnesium under fully
anaerobic conditions would significantly affect the rates of
enzymatic reactions28. The concentration of ionized
magnesium inside red cells is not therefore constant, but
continually oscillates as the cells circulate.
Measurement of cytosolic free magnesium ion concentration by
19F NMR
To avoid some of the problems with 31P-NMR, several workers
have successfully used exogenous NMR magnesium indicators to
determine magnesium29. The cells are suspended in a
loading buffer containing acetoxymethyl ester of MG-APTRA. 19F
NMR studies of 4-methyl-5fluoro-APTRA-loaded human erythrocytes
indicate a basal free magnesium level of 0.25 mM. The lack of
change in cytosolic free magnesium upon raising the extracellular
magnesium to 6 nM is consistent with the data of others
suggesting that intracellular magnesium is slow to exchange or
equilibrate with extracellular magnesium.
Conclusion
To our knowledge, ionized magnesium content of human intact
erythrocytes has been measured mainly by the NMR method or by
zeropoint titration. Both microelectronics and Mag-Fura measure
the activity of magnesium ions, not the concentration.
As with most of the fluorescent indicators, one needs to be
concerned about changes in autofluorescence, and indicator
leakage from the cell. The excitation maximum for magnesium
complexed furaptra is 335 nm, thus overlapping with cell NADH
fluorescence. Thus, controls should be done in cells without
indicators to assure that changes in fluorescence attributed to
furaptra are not significantly contributed to by changes in NADH.
Also the presence of the chelator in the cytoplasm of the cell
increases the cell buffering capacity for that ion. One also
needs to be sure that the addition of the chelator to the cell
does not alter cell function. While fluorescent probes offer the
advantage of ease of use and the possibility of routine advantage
of ease of use and the possibility of routine measurement,
neither MagFura-2 nor Mag-Fura-5 are ideally suited for the study
of intracellular magnesium regulation, because of calcium and pH
sensitivity, respectively. Fluorescence is a highly sensitive
technique that can detect concentrations as low as
10-8 M, as opposed to 10-4 M for NMR
spectroscopy. Because of the high sensitivity of fluorescence,
only 5 µM of furaptra is required for loading, as opposed
to 50 µM of the 19 F NMR indicators; thus, toxicity effects
are minimized.
Ionized magnesium in erythrocytes has been measured in 14
healthy controls with both 31P NMR and zero point titration
30. The concentration of ionized magnesium as
estimated by NMR was significantly lower than measured by
zeropoint titration, but no significant correlation could be
detected between the erythrocyte ionized magnesium levels
estimated with these two methods. (Mg2+)i
(mean ± ISD) measured with the 31P NMR method (mmol./litre
cell wt) was found to be 0.20 (0.03) and with the zeropoint
titration (mmol/litre cell wt) 0.55 (0.12). The NMR and zeropoint
titration methods gave different values for ionized magnesium
concentration in human erythrocytes, which showed no correlation.
Thus the uncertainty concerning the two currently used techniques
for free magnesium determination in erythrocytes (i.e.,
NMR and zeropoint titration methods) is worsened by the fact that
ionized magnesium inside red cells continually oscillates in
vivo.
Alterations in magnesium binding during cold storage of
erythrocytes
Intracellular free magnesium concentration falls to one-third
of its original level after 11 days storage at 4° C in
standard blood preservation media. The fall occurs in spite of
little change in the total magnesium content of the cells,
indicating a change in the buffering characteristics of the
cytoplasm 31. Binding of magnesium to ligands other
than ATP and 2,3-bisphosphoglycerate must increase during
storage. Storage did not significantly affect basal or
sodium-stimulated efflux of magnesium from magnesium-loaded red
cells. During storage the degree of magnesium binding
attributable to ATP and DPG declines. Calcium but not magnesium
permeability of human red cells increases during cold storage
32.
Control of erythrocyte magnesium
In vitro study of the control of erythrocyte
magnesium
Introduction
In order to study magnesium homoeostasis, it is necessary to
know the ionized as well as the total magnesium concentration
since it is ionized magnesium that is regulated by transport
systems. Plasma ionized magnesium concentration is about 0.5 mM
and the membrane potential in erythrocytes is about 9 mv, inside
negative. These values predict that intracellular ionized
magnesium concentration should be about 1 mM at electrochemical
equilibrium. In fact measured values in red cells are all well
below this level. Analysis of the ionized intracellular magnesium
concentration (Mg2+)i also shows whether or
not magnesium is at equilibrium across the membrane. Red cell
magnesium permeability appears to be very low. It has been
suggested in human studies that any 28Mg entering the
cells did so during early phases of erythropoeisis. The
observation that magnesium can move across the human red cell
membrane prompted careful exploration of the fluxes from cells
with normal magnesium content into media containing sodium but
nominally free of magnesium. Fluxes ranging from about 4-7
µmol/litre/cell/h were detected after correction for
haemolysis. Although these fluxes are small, they are much higher
than would be expected from passive diffusion through the lipid
of the membrane33.
Intracellular free and bound magnesium represent a magnesium
buffer system. If the capacity of the intracellular magnesium
buffer is exhausted, constant pMgi is supported by net
magnesium influx or net magnesium efflux across the plasma
membrane. Moreover, magnesium uptake may occur without
significant changes in free magnesium because of buffering of
intracellular magnesium34.
Sodium-dependent magnesium efflux
When rat or chicken erythrocytes are loaded with magnesium by
incubation of the cells at increased
(Mg2+)o in the presence of the divalent
cation ionophore A23187, the erythrocytes take up magnesium.
Magnesium efflux took place only when
(Mg2+)i was increased and stopped when the
physiological magnesium content was reached. Magnesium efflux was
energy-dependent and reduced by reduction of intracellular ATP in
magnesium-loaded human erythrocytes. In vitro, magnesium
efflux was specifically dependent on (Na+).
Substitution of (Na+)o by K+,
L+ or Choline+ could not support magnesium
efflux. Magnesium efflux was stoichiometrically coupled with the
uptake of extracellular sodium: with human erythrocytes a ratio
of 3 sodium to 1 magnesium as reported, in analogy to the
Na+/Ca2+ antiport at the cell membrane.
Magnesium efflux was irreversible. When erythrocytes were
magnesium depleted, loaded with sodium and incubated at elevated
(Mg2+)o in the presence of ATP, there was
no magnesium uptake. Irreversibility of
Na+/Mg2+ antiport only holds for net
magnesium efflux.
In chicken erythrocytes 28Mg2+ uptake
occurs in exchange for non-radioactive intracellular
24Mg2+. The 28Mg2+ -
24Mg2+ exchange in loaded and non-loaded
cells showed the same inhibition by amiloride and
(Na+)o. Therefore it can be concluded that
in magnesium-loaded and non-loaded cells the magnesium efflux
system exchanges intracellular for extracellular magnesium. In
this exchange system extracellular sodium competes with
extracellular magnesium. After magnesium loading the magnesium
exchanger has changed its properties, leading to
Na+/Mg2+ antiport. This effect is an
analogy to the function of Na+/H+ antiport
which becomes active at increased (H+)i. It
can be concluded that in the presence of high
(Mg2+)i, a higher portion of
Mg2+ -Mg2+ exchange occurs simultaneously
with Na+/Mg2+. In rat erythrocytes
(Na+)i competes with (Mg2+), for
Na+/Mg2+ antiport. All these results were
obtained from rat or chicken erythrocyte experiments. In chicken,
rat, and human erythrocytes Na+/Mg2+
antiport was ATP-dependent.
Sodium-independent magnesium efflux
In magnesium-loaded human erythrocytes a major fraction of
magnesium efflux occurred in sodium-free potassium medium.
Sodium-independent magnesium efflux from magnesium-loaded human,
rat and chicken erythrocytes was the highest in sucrose medium
and reduced to the same degree in KCl, LiCl and choline chloride.
Sodium-independent magnesium efflux was reduced by high
(Cl)o and by
(4,4'-diisothiocyanatostilbene-2,2-disulphonicacid) DIDS.
From these results it was concluded that sodium-independent
magnesium efflux functions in combination with net Cl-
efflux for charge compensation. Magnesium efflux from human
magnesium-loaded erythrocytes was found (mmol/litre cells*30 min)
in sucrose (sodium-independent 0.89, in choline chloride
(sodium-independent) 0.33 and in NaCl-choline chloride
(sodium-dependent) 0.16). Sodium-dependent magnesium efflux from
human, rat and chicken erythrocytes can be partly inhibited by
serum albumin34. In experiments with
magnesium-depleted erythrocytes, no significant reuptake of
magnesium was found. Only when reticulocytes were
magnesium-depleted could reuptake of magnesium be observed. In
erythrocytes there is an additional magnesium efflux operating in
combination with Cl efflux for charge compensation.
In vivo study of the control of the red cell
magnesium
Genetic regulation of cellular magnesium content
Major differences in erythrocyte (Er) magnesium content have
been shown to exist between different ethnic groups35.
Mean erythrocyte magnesium values were 20-30 per cent lower in
black African men than in Amerindian Quechuas of the same sex and
age. European Caucasians exhibited intermediate values. These
differences appeared to be relatively stable and partly
independent of the climate in which the subjects were residing.
In a given population, large differences between subjects were
also observed. Healthy adult male blood donors examined in Paris
revealed a 65 per cent difference between the lowest value (1.55
mmol/litre of erythrocytes) and the highest one (2.89 mmol).
Large and fairly stable interethnic and interindividual
variations led Henrotte35 to propose the hypothesis of
a genetic control of the erythrocyte magnesium level in humans.
However, the relative contribution of genetic vs
environmental factors was a priori difficult to assess.
The existence of a genetic regulation of erythrocyte magnesium
content has now been fully confirmed. By comparing monozygotic
twins living together and apart, Darin et
al.36 were able to discriminate the genetic
component from the familial non-genetic component. Environmental
factors that begin to differ after twins have been separated, had
some influence on the plasma magnesium and none (or very little)
on the erythrocyte magnesium values. There is a very significant
association between erythrocyte magnesium and HLA-B antigens. The
largest associated variations with HLA-B antigens (between
B35+ Bw6+ and B38+ subjects) are
equal to 10 per cent of the total population erythrocyte
magnesium mean. The association between erythrocyte magnesium and
HLA is much stronger in men than in women, suggesting the
interaction of sex-linked factors; one of these factors could be
the larger variability of erythrocyte magnesium in women with
age, menstrual cycle and oral contraceptive intake.
Thus, erythrocyte magnesium level is controlled by genetic
factors. In erythrocytes, other phenomena may hamper the
interpretation of results. Erythrocyte magnesium levels decrease
with cell age: high levels in reticulocytes decrease and reach a
steady state in mature erythrocytes and decrease again in older
cells. Genetically low erythrocyte magnesium values could,
therefore, be ascribed to the older age of the erythrocyte
population. The interpretation of the genetic regulation of cell
magnesium is also hindered by the fact that the total
intracellular magnesium level represents both bound and free
magnesium contents. A statistically significant correlation has
been found between free and total magnesium
contents37.
Neurohormonal regulation of cellular
magnesium38
A complex neuro-hormonal system is instrumental in the control
of the stability of intracellular magnesium and of the
consequences of magnesium status disturbances. Exchanges between
extracellular compartments and soft tissues elicit secretion of
hormones and neurohormones: adrenalin and insulin, taurine (and
perhaps glutamyltaurine), and finally beta-stimulation of the
adrenergic receptors.
These neuro-hormonal factors regulating cellular magnesium
content are very important to appreciate the significance of the
variations of erythrocyte magnesium concentrations in case of
several pathological or iatrogenic disturbances such as diabetes
mellitus, phaeochromocytoma or stress
pathology38-40.
Conclusion
It should be emphasized that variations in the concentration
of total or ionized magnesium in a given cell do not necessarily
mean that similar changes should exist in other cellular
concentrations or in the magnesium pool. Changes may occur in the
course of disturbances in the distribution of the ion and
constitute evidences of functional or organic disorders in the
analysed cell.
It remains that the basic cellular magnesium item for
evaluating the magnesium status is the static measurement of
erythrocyte magnesium which once again has been experimentally
and clinically validated in the investigation of primary chronic
marginal magnesium deficiency. Its value will be subsequently
enhanced when it takes its place among the clinical and
paraclinical data of dynamic investigations41-44.
References
1. Martin M.T. & Shapiro, R. (1988): Atomic absorption
spectrometry of magnesium. Methods Enzymol.
158, 365-370.
2. Sturgeon, R.E. & Berman, S.S. (1983): Determination of
the efficiency of the graphite furnace for atomic absorption
spectrometry. Anal. Chem. 55,
190-200.
3. Harrison, W.W. & Wadlin, W.H. (1969): Magnesium spinell
interferences in air-acetylenevs nitrous oxide-acetylene
flames in atomic absorption spectrometry.
4. Elwell, W.T. & Gidley, A.F. (1961): In: Atomic
absorption spectrophotometry, pp. 67-75. New York:
Macmillan.
5. Ramakrishna, T.V., Robinson, J.W. & West, P.W. (1966):
The determination of calcium and magnesium by atomic absorption
spectroscopy. Anal. Chim. Acta 36,
57-64.
6. Halls, D.J. & Townshend, A. (1966): A study of some
interferences in the atomic absorption spectrophotometry of
magnesium. Anal. Chim. Acta 36,
273-278.
7. Willis, J.B. (1960): The determination of metal in blood
serum by atomic absorption spectroscopy. II. Spectrochim
Acta 16, 273-278.
8. Price, W.J. (1972): In: Analytical atomic absorption
spectrometry, pp. 163-166. New York: I Heyden.
9. Stendig-Lindberg, G., Penciner, J., Rudy, N. & Wacker,
W.E.C. (1984): Comparison of diluents for serum magnesium.
Magnesium 3, 50-56.
10. Lijnen, P., Hespel, P., Lommelen, G., Laermans, M.,
M'Buyamba-Kabangu, J.R. & Amery, A. (1986): Intracellular
sodium, potassium and magnesium concentration, ouabain-sensitive
ribidium-uptake and sodium-efflux and
Na+,K+ cotransport activity in erythrocytes
of normal male subjects studied on two occasions. Meth. Find.
Exptl. Clin. Pharmacol. 8, 525-533.
11. Fisher, P.W.F., Belonje, B. & Giroux, A. (1993):
Magnesium status and excretion in age-matched subjects with
normal and elevated blood pressure. Clin. Biochem
26, 207-211.
12. Cameron, I.L., Hardman, W.E., Smith, N.K.R., Fullerton,
G.D. & Miseta, A. (1993): Changes in the concentration of
ions during senescence of the human erythrocyte. Cell.
Biol. Int. 17, 93-98.
13. Tongyai, W., Rayssiguier, Y., Motta, C., Gueux, E.,
Maurois, P. & Heaton, F.W. (1989): Mechanism of increased
erythrocyte membrane fluidity during magnesium deficiency in
weanling rats. Am. J. Physiol. 257,
C270-C276.
14. Rousselet, F. & Durlach, J. (1971): Méthodes
analytiques et exploration pratique du metabolisms du magnesium
en clinique
humaine. Symposium on magnesium deficit in human
pathology, Vol. 1, p. 65-90. Vittel.
15. Duester, P.A., Trostman, U.H., Bernier, L.L. & Dolev,
E. (1987): Indirect vs direct measurement of magnesium
and zinc in erythrocytes. Clin. Chem.
33/34, 529-532.
16. Bonnay, M.M., Loegac, E.M., Brunier, A.P. & Bohuon,
C.J. (1988): Intra-erythrocytic Mg assay in the Kodak Ektachem
700 analyser. Clin. Chem. 34,
2154-2155.
17. Moretto, P., Llabador, Y., Simonoff, M., Bara, M.,
Guiet-Bara, A. & Durlach, J. (1993): The nuclear microprobe:
a powerful microanalysis technique for magnesium and trace
element research. In: Health and disease (role of magnesium
and trace minerals), eds. R. Nath & K.D. Gill, pp.
93-101, New Delhi: Ashish Publishing.
18. Schuette, S., Vereault, D., Ting B.T.G. & Janghorbani,
M. (1988): Accurate measurement of stable isotopes of magnesium
in biological materials with inductively coupled plasma mass
spectroscopy. Analyst 113,
1837-1842.
19. McGuigan, J.A.S., Buri, A., Chen, S., Illner, H. &
Lilthi, S. (1993): Some theoretical and practical aspects of the
measurement of the intracellular free magnesium concentration in
heart muscle: consideration of its regulation and modulation.
Magnesium and the cell. ed. N.J. Birch, pp. 91-120.
London: Academic Press,
20. Rose, I.A. (1968): The state of magnesium in cells as
estimated from the adenylate kinase equilibrium. Proc. Natl.
Acad. Set. USA 61, 1079-1086.
21. Berger, H., Jänig, G.R., Gerber, G., Ruckpaul, K.
& Rapoport, S.M. (1973): Interaction of haemoglobin with
ions, Eur. J. Biochem. 38, 553-562.
22. Spichiger, U.E., Eugster, R., Schaller, U., Haase, E.
& Simon, W. (1991): Magnesium-selective electrodes: state of
the art. Magnes. Bull. 13, 140-144.
23. Mota de Freitas, D. & Dorus, D. (1993): Techniques for
measuring magnesium in tissues from hypertensive, psychiatric and
neurological patients. In: Magnesium and the cell ed.
N.J. Birch, pp. 51-79. London: Academic Press.
24. Murphy, E. (1993): Measurement of intracellular ionized
magnesium. Miner. Electrolyte Metab. 250-258.
25. Flatman, P. & Lew, V.L. (1977): Use of ionophore
A23187 to measure and control free and bound cytoplasmic Mg in
intact red cells. Nature 267,
360-362,
26. Flatman, P.W. (1980): The effect of buffer composition and
deoxygenation on the concentration of ionized magnesium inside
human red blood cells. J. Physiol. 300,
19-30.
27. Alvarez-Leefmans, F.J., Giraldez, F. & Gamino, S.M.
(1987): Intracellular free magnesium in excitable cells: its
measurement and its biologic significance. Can. J. Physiol.
Pharmacol. 65, 915-925.
28. Gupta, R.K., Benovic, J.L. & Rose, Z.B. (1978): The
determination of the free magnesium level in the human red blood
cell by 31P NMR. J. Biol. Chem. 253,
6172-6176.
29. Levy, L.A., Murphy, E., Raju, B. & London, R.E.
(1988): Measurement of cytosolic free magnesium ion concentration
by 19F NMR. Biochemistry 27,
4041-4048.
30. Geven, W.B., Vogels-Mentink, G.M., Willems, J.L., Hilbers,
C.V., Os, C.V. & Monens, L.A.H. (1991): Ionized Mg content of
human erythrocytes measured by the NMR method compared with
values obtained by zeropoint titration. In: Magnesium- a
relevant ion, eds. B. Lasserre & J. Durlach, pp.
291-293. London: John Libbey.
31. Flatman, P.W. (1988): The control of red cell magnesium.
Magnes. Res. 1, 5-11.
32. Bock, J. L. & Yusuf, Y. (1988): Further studies on
alterations in magnesium binding during cold storage of
erythrocytes. Biochem. Biophys. Acta
941, 225-231.
33. Flatman, P.W. (1991): Mechanisms of magnesium transport.
Annu. Rev. Physiol. 53, 259-271.
34. Vormann, J. & Günther, T. (1993): Magnesium
transport mechanisms. In: Magnesium and the cell, ed.
N.J. Birch, pp. 137-155. London: Academic Press.
35. Henrotte, J.G. (1993): Genetic regulation of cellular
magnesium content. In: Magnesium and the cell, ed. N.J. Birch,
pp. 137-155. London: Academic Press.
36. Darin, P., Michotte, Y., Defrise-Gussenhoven, F. &
Henrotte, J.G. (1981): The inheritance of plasma and red blood
cell magnesium and zinc levels studied from twin and family.
Acta. Genet. Med. Gemell. 30,
67-50.
37. Santarromana, M., Delepierre, M., Ferray, J.C., Franck,
G., Garay, R. & Henrotte, J.C. (1989): Correlation between
total and free magnesium levels in human red blood cells.
lnfluence of HLA antigens. Magnes. Res.
2, 281-283.
38. Durlach, J. (1988): Regulation of cellular magnesium. In:
Magnesium in clinical practice, ed.. J. Durlach, pp,
31-40. London: John Libbey.
39. Durlach, J. (1988): Secondary magnesium deficits;
magnesium overload. In: Magnesium in clinical practice,
ed. J. Durlach, pp. 114-215. London: John Libbey.
40. Classen, H. G. (1990): Systemic stress and the role of
magnesium. In: Metal ions in biological systems, vol. 26.
Compendium on magnesium , eds. H. Sigel & A. Sigel, pp.
321-329. New York: Marcel Dekker.
41. Durlach, J. (1988): Methods of evaluating magnesium
status. In: Magnesium in clinical practice, ed. J.
Durlach, pp. 40-60. London: John Libbey.
42. Durlach, V., Millart, H., Meer, L., Grulet. H,, Gross, A.
& Leutenegger, M. (1991): Magnesium status in a group of
so-called 'spasmophilic patients'. Magnes. Res.
4, 233--234.
43. Nowitzki-Grimm, S., Grimm, P., Thoni, H. & Classen,
H.G. (1991): Diagnosis of magnesium, status. Magnes.
Bull. 13, 107-115.
44. Beretta, P., Ambrosini, G., Concari, M. & Bargellini,
A. (1993): Is magnesium content in erythrocytes suitable for
evaluating cation retention after oral physiological
supplementation in marginally magnesium deficient subjects.
Magnes. Res. 6, 149-153.
All articles by Dr. Durlach are copyrighted, and permission is
granted to Web users only to make single hard copies for personal
use. Additional reprints should be obtained from the originating
journals. Excerpts may be used by the media with attribution to
Dr. Durlach.
This page was first uploaded to The Magnesium Web Site on
April 29, 1996
http://www.mgwater.com/