Magnesium Research (1994) 7, 1, 11-16
Experimental paper
Comparative effects of MgCl2 and MgSO4
on the ionic transfer components through the isolated human
amniotic membrane
1Michel Bara,1Andrée Guiet-Bara
and 2Jean Durlach
1Physiopathology of Development: Cellular
Interactions, Université P.M. Curie, 4 Place Jusseiu,
75252 Paris Cedex 05, France; 2SDRM, Hôpital St.
Vincent de Paul, 74-82 Rue Denfert Rochereau, 75014 Paris,
France
Summary: The effects of
MgCl2 and MgSO4 are different on the total
transfer through the human amniotic membrane: MgCl2 at
low concentration (1 mM) decreases the total conductance Gt and
increases it at high concentration (4 mM) on the fetal side (FS)
and on the maternal side (MS), while MgSO4 has no
effect on the MS and increases Gt on the FS. Moreover, whatever
the concentration, MgCl2 increases the flux ratio
F1/F2 while MgSO4 decreases it to reach a value near
to 1. Gt is the sum of various components: three paracellular
components (Gp) and nine cellular components (Gc constituted from
channels, exchangers, antiporters and cotransporters). All
components of Gt on the two faces, are decreased by 1 mM
MgCl2 and increased at 4 mM. MgCl2 also has
an effect on all ionic exchangers across the membrane. In
contrast, on the MS, MgSO4 (1mM) decreases GpNa,
increases GpK and the antiport Na/H component and has no effect
on any of the other components, while at 4 mM, MgSO4
has no effect. On the FS, MgSO4 (1 mM) increases GpNa
and GpK, but does not modify the other components. At 4 mM, the
effect is the same, except for an increase of GpCl. These data
show the importance of the anion-cation association in the ionic
exchanges through a membrane: MgCl2 and
MgSO4 have a different action --MgCl2
interacts with all the exchangers, while the effect of
MgSO4 is limited to paracellular components without
interaction with cellular components excepted the antiport
Na/H.
Key words: Amniotic membrane,
conductance components, exchangers, MgCl2,
MgSO4.
Introduction
Among numerous magnesium salts, MgCl2 and
MgSO4 are the ones most commonly used in magnesium
therapy. Curiously, the most widely used preparations are those
containing magnesium sulphate. However, among the soluble salts
of magnesium, this salt has the least advantageous
pharmacological properties. Its absorption, cellular penetration,
membrane effects and anti-hypoxic effects are poor1-3.
Comparative studies between MgCl2 and MgSO4
indicate classically a better absorption for MgCl2
than for MgSO44-8 and a better retention
for MgCl23,9,10. Moreover, sulphate
infusion leads to a considerable increase in the urinary
excretion of magnesium11, calcium and potassium
12,13. The membranous effects of magnesium salts may e
evaluated by the study of monovalent fluxes. It has already been
shown that the effects of MgCl2 and MgSO4
are different on the total transfer through the human amniotic
membrane14-18: MgCl2 at low concentration
(1 mM) decreases the total conductance, Gt, and increases it at
high concentration (4 mM) on the fetal side (FS) and on the
maternal side (MS), while MgSO4 has no effect on the
MS and increases Gt on the FS. Moreover, whatever the
concentration, MgCl2 increases the fluxes ratio F1/F2,
while MgSO4 decreases it to reach a value near to 1.
Gt is the sum of various components: three paracellular
components (Gp) and nine cellular components (Gc, constituted
from ionic channels, exchangers, antiporters and cotransporters),
and the aim of this work was to study the comparative effects of
MgCl2 and MgSO4 on all components of
amniotic transfer to identify the targets of the deleterious
action of MgSO4.
Material and methods
Membrane preparation
Strips of human amnion, isolated from the amniotic sac, were
obtained after 12 normal full term deliveries. The amnion was
immersed into Hanks' solution (pH 7.4 and 37 ± 1° C).
A circular area of the amnion was sampled and placed between two
acrylic chambers containing Hanks' solution according to the Bara
and Guiet-Bara device19.
Electrical parameters
The cellular conductance (Gc) in the maternal-to-fetal and in
the fetal-to-maternal directions was measured by injecting a
constant current square wave pulse through a double-barrelled
microelectrode and analysing the recorded transient voltage on an
oscilloscope. The transamniotic ionic conductance, Gt, was
measured by observing the transamniotic potential difference when
a direct current was passed across the whole tissue. The
transamniotic potential difference was recorded with two
agar-agar salt bridges placed 1.3-1.5 mm from each side of the
tissue, while an electrical current was passed across the tissue
by means of Ag/AgCl electrodes and agar-agar salts and measured
on a Schlumberger electrometer. The paracellular conductance Gp =
Gt - Gc.
Metabolic inhibitors
Various metabolic inhibitors, which were used to observe the
different components of the total transamniotic conductance,
were20:
(1) Cellular conductance - Ouabain (0.1) mM);
Na/K-ATPase (Ge1); amiloride (0.5 mM); Na+ channels
(GlNa); bumetanide (0.1 mM); Na-K-2Cl cotransport (Ge3); barium
(1 mM); K+ channels (G1K); diisothiocyanostilbene
disulphonic acid (300 µM); Cl- channels (GlCl);
acetamido-isothio-cyanostilbene-disulphonic acid (300 µM)
Cl-HCO3 antiport (Ge4); manganese (1 mM); Na-Mg
exchanger (Ge5); dinitrophenol (20 mM): coupling factor
(Gcp).
(2) Paracellular conductance - Triaminopyrimidinium
(10 mM); GpNa; protamines (100 mg/litre); GpK and GpCl.
Solutions
The composition of the Hanks' solution used in the experiments
was (mmol/litre): NaCl 150, KCl 6, CaCl2 1,
MgCl2 0.5 MgSO4 0.5,
Na2HPO4-KH2PO4-NaHCO3
1, glucose 5.5. The magnesium (MgCl2 or
MgSO4) was added on the maternal or fetal sides of the
amnion at two concentrations: 1 mM and 4 mM.
Statistical analysis
The results are expressed as means ±SD. Statistical
comparisons were carried out by conventional paired data
analysis.
Results
Effects of MgCl2
Paracellular components
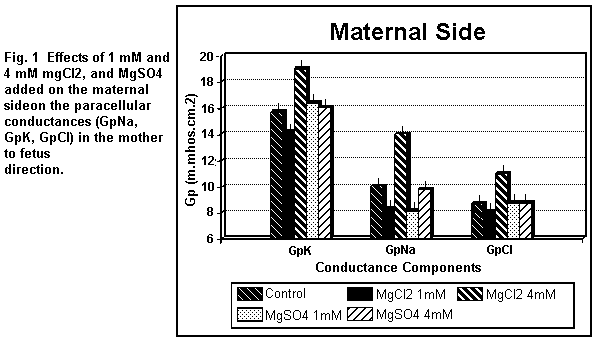
On the maternal side (Fig. 1), the addition of 1 mM
MgCl2 induced a decrease (P < 0.01) in all the
paracellular components (GpK, GpNa, GpCl); at 4 mM, the three
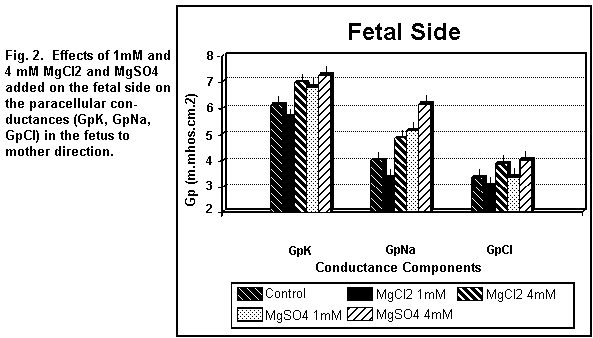
components were increased (P < 0.01). On the fetal side (Fig.
2), the addition of 1 mM MgCl2 decreased (P < 0.01)
GpK, GpNa and GpCl; at 4 mM, the three components were all
increased (P < 0.01).
Cellular components
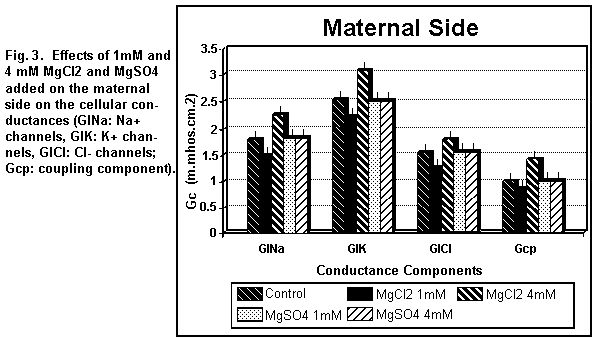
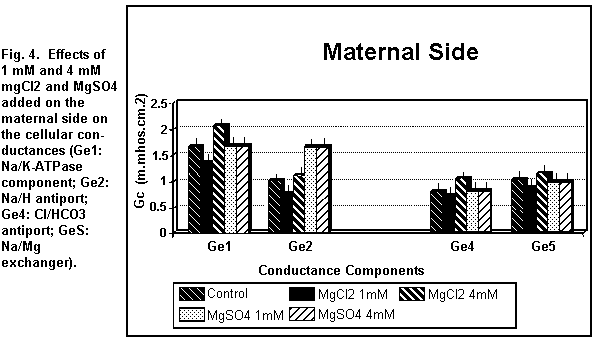
On the maternal side (Figs. 3 and 4), the addition of 1 mM
MgCl2 induced a decrease (P < 0.01) in all the
cellular components; at 4 mM, all components were increased (P
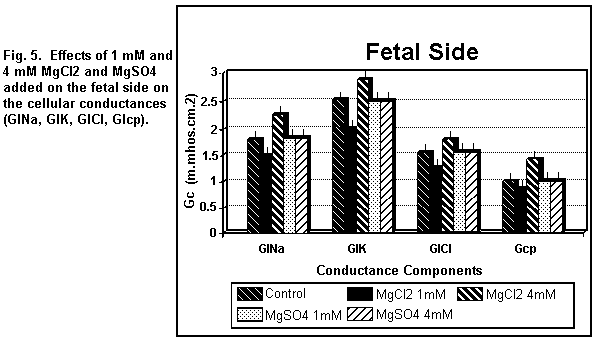
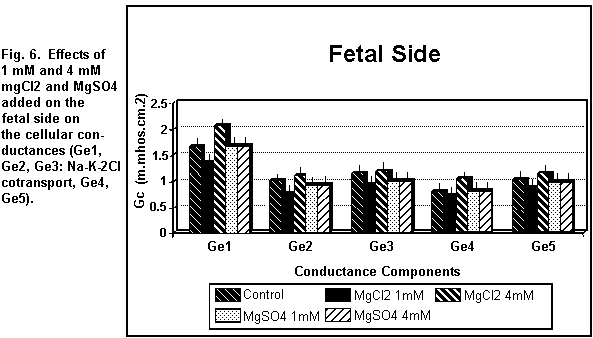
< 0.01). On the fetal side (Figs. 5 and 6), the addition of 1
mM MgCl2 decreased (P < 0.01) all the cellular
components significantly; at 4 mM, there was an increase in all
the components (P < 0.01).
Effects of MgSO4
Paracellular components
On the maternal side (Fig. 1), the addition of 1 mM
MgSO4 induced an increase (P < 0.01) in GpK, and a
significant decrease in GpNa, but had no effect (P = 0.25) on
GpCl; at 4 mM, GpK, GpNa and GpCl remained constant (P = 0.3). On
the fetal side (Fig. 2), the addition of 1 MgSO4
induced an increase (P <0.01) in GpK and GpNa and had no
action (P = 0.20) on GpCl; at 4 mM, the three components were all
increased (P < 0.01).
Cellular components
On the maternal side (Figs. 3 and 4), the addition of 1 or 4
mM MgSO4 had no significant effect (P=0.25) on any of
the cellular components except the antiport Na/H component (Ge2)
which was increased (P < 0.01). On the fetal side (Figs. 5 and
6), the addition of 1 or 4 mM MgSO4 had no significant
effect (P = 0.30) on any of the cellular components.
Discussion
The biological effects of Cl- and SO4 2-
are generally opposite. For example, following infusion of
hypertonic sodium sulphate, increases in serum Na concentration
and decreases in serum chloride, potassium, phosphate, calcium
and magnesium were regularly observed12. In general,
studies of the membrane effects of MgCl2 and
MgSO4 have shown that the sulphate anion has
unfavourable effects in comparison with other ions used in
magnesium therapy. Our previous studies14-18 on the
membrane effects of MgCl2 and MgSO4 on
monovalent ion fluxes have shown that MgSO4 has
deleterious actions in comparison with other magnesium salts,
particularly MgCl2. These data may be compared to the
effects of waters containing sulphuric acid or sulphate ions on
cardiovascular diseases--the presence of SO4 in water
may act as a cardiovascular risk factor21,22.
In the present study, we have shown that on the maternal and
fetal sides MgCl2 decreases all components at 1 mM and
increases them at 4 mM, according to the biphasic effect already
observed on the total conductance Gt 23. In contrast,
MgSO4 has a differential action on the two sides. On
the MS, 1 mM MgSO4 decreases GpNa, increases GpK and
the antiport Na/H, and has no effect on the other components,
while at 4 mM, MgSO4 has no significant effect on any
components except for an increase in the antiport Na/H exchange.
On the FS, 1 mM MgSO4 increases GpNa and GpK and does
not modify the other components. At 4 mM, the effect is the same
except for an increase of GpCl. Also, the effects of
MgSO4 are limited to the paracellular components and
to the antiport Na/H.
MgSO4 does not act on the Na/K-ATPase component
(Ge1). This result implies that the hypothesis of a direct
contribution of MgSO4 to the membrane potential is
inaccurate. On the other hand, MgCl2, which interacts
with the Na/K-ATPase component, may participate directly in the
formation of the membrane potential. Moreover, MgCl2,
which increases the ionic channel conductances (G1Na, GlK, GlCl),
regulates the viability of these channels and may decrease or
increase the permeability of monovalent ions as a function of its
concentration. This is not true for MgSO4. The
principal effect of MgSO4 is on the paracellular
conductances which are structurally situated in the intercellular
space.
It is possible to explain the results obtained with
MgCl2 and MgSO4 by their crystal
structures24. MgCl2 forms a stable
hexahydrate [Mg(H2O)6 Cl2] between -3.4 and +116°
C; the crystals consist of dianions with the magnesium
co-ordinated to the six water molecules as a complex,
[Mg(H2O)6]2+, and two independent chloride anions,
Cl-. MgSO4 forms a stable heptahydrate
[Mg(H2O)7 SO4] at ambient temperature; the
crystals again consist of dian-ions with the magnesium
coordinated to six water molecules, while the seventh water
molecule is associated with the sulphate anion:
[Mg(H2O)6]2+ [SO4+H2O]2-.
Therefore both salts are hexa-aqueous complexes of magnesium and
the counter ions (Cl-,SO4 2-) do not form
complexes with magnesium ions. Moreover, MgCl2
measures about 12 per cent magnesium, while MgSO4
measures about 10 per cent magnesium3.
These data confirm the important role of the anion and may
explain the present results. The size of MgCl2 (a
smaller molecule than MgSO4 should allow interactions
with the various exchange components (paracellular and cellular),
and the more hydrated MgSO4 molecule implicates
bindings with external charges, that is, interactions with
paracellular components in preference to cellular components. In
the two crystals, magnesium has the same complex form
([Mg(H2O)6]2+). The different effects observed might
also be attributed to the anions associated with magnesium.
This observation is important in clinical practice. For
example, the hypocalcaemia observed during high dose magnesium
therapy should not only be interpreted as an effect of magnesium
overload on the calcium metabolism, but also in relation to the
particular effects of the anion used on the calcium metabolism
(SO4 increases the calcium urinary excretion
12,13.
Conclusion
MgCl2 and MgSO4 have differential
effects on the ionic exchange through the human amniotic membrane
(MgCl2 interacts with paracellular and cellular
transfer; MgSO4 interacts with paracellular transfer
only). These data show the importance of the anion-cation
association. During magnesium therapy, it is important to
consider the anion effect associated with magnesium.
References
1. Duhm, J., Deuticke, B. & Gerlach, E. (1968): Metabolism
of 2,3-diphosphoglycerate and glycolysis in human red blood cells
under the influence of dipyramidole and inorganic sulfur
compounds. Biochim. Biophys. Acta 170,
452-454.
2. Morris, M.E. & Levy. G. (1983): Absorption of sulfate
from orally administered magnesium sulfate in man. J.
Toxicol. Clin. Toxicol. 20, 107-114.
3. Durlach, J. (1988): Magnesium in clinical
practice, 360 pp. London, Paris: John Libbey.
4. Richard, A. & Hazard, R. (1943): Précis de
Thérapeutique et de Pharmacologie. p. 3856. Paris:
Masson.
5. Glénard, R. (1948): Le magnésium et la fibre
lisse. In: Journées thérapeutiques de
Paris 1947, pp. 313-320. Paris: Doin.
6. Classen, H.G.. Marquardt, P., Späth, M., Ebel, H.
& Schumacher, K.A. (1973): Vergleichende tierexperimentelle
Untersuchungen über die Resorption von Magnesium also
Sulfate, Chlorid, Aspartat und Aspartat-Hydrochlorid aus dem
Magen-Darm-Trakt. Arzneimittelforschung
23, 267-271.
7. Classen, H.G. (1990): Magnesium and placebo effects in
human medicine. In: Metal ions in biological systems,
vol. 26, eds. H. Sigel & A. Sigel, pp. 597-609. New York:
Marcel Dekker.
8. Malagelada. J. R., Holtermuller, K.H., McCall, J.T. &
Vay Liang, W.G. (1978): Pancreatic gallbladder and intestinal
responses to intraluminal magnesium salts in man. Am. J. Dig.
Dis. 23, 481-485.
9. Franke, H. (1934): Magnesium und Kohlehydratstoffwechsel.
Arch. Exp. Pathol. Pharmacol. 174,
727-740.
10. Naito, Y. (1980): Effect of Mg salts on urinary Mg
excretion in rats fed hypomagnesic diet. Igaku to
Seibutsugaku 101, 173-176.
11. Martin, H.E., Mehl, J. & Wertman, M. (1952): Clinical
studies of Mg metabolism. Med. Clin. North Am.
36, 1157-1171.
12. Wolf, A.V. & Ball, S.M. (1950): Effect of intravenous
sodium sulfate on renal excretion in the dog. Am. J.
Physiol. 160, 353-360.
13. Walser, M. & Browder, A.A. (1959): Ion association.
III. The effect of sulfate infusion on calcium excretion. J.
Clin. Invest. 38, 1404-1411.
14. Bara, M. Guiet-Bara, A. & Durlach, J. (1984):
Comparative effects of MgCl2 and MgSO4 on
monovalent cations transfer across isolated human amnion.
Magnes. Bull. 6, 36-40.
15. Guiet-Bara, A., Bara, M. & Durlach, J. (1985):
Cellular and shunt conductances of human isolated amnion. II.
Comparative effects of MgCl2 and MgSO4:
electrophysiological studies. Magnes. Bull.
7, 16-19.
16. Bara, M.. Guiet-Bara. A. & Durlach, J. (1988):
Modification of human amniotic membrane stability after addition
of magnesium salts. Magnes. Res. 1,
23-28.
17. Bara, M., Guiet-Bara, A. & Durlach, J. (1988):
Analysis of magnesium membraneous effects: binding and screening.
Magnes. Res. 1, 29-33.
18. Durlach, J., Durlach, V., Bara, M., Guiet-Bara, A. (1992):
A new method of in vitro prescreening evaluation of
several Mg salts. Meth. Find. Exp. Clin. Pharmacol.
14, 305-310.
19. Bara. M. & Guiet-Bara. A. (1981): Détermination
des constantes électriques de la membrane des cellules
épithéliales amniotiques humaines in
vitro. C.R. Soc. Biol. 176,
749-754.
20. Bara, M., Guiet-Bara. A. & Durlach. J. (1990):
Comparative study of effects of magnesium and taurine on
electrical parameters of natural and artificial membranes. VII.
Effects on cellular and paracellular ionic transfer through
isolated human amnion. Magnes. Res. 3,
249-254.
21. Durlach, J., Bara, M. & Guiet-Bara, A. (1985):
Magnesium level in drinking water and cardiovascular risk factor:
a hypothesis. Magnesium 4, 5-15.
22. Kobayashi, J.A. (1957): Geographical relationship between
the chemical nature of river water and death rate from apoplexy.
Ber. Ohara Inst. Lansw. Biol. 11,
12-21.
23. Bara, M., Guiet-Bara, A. & Durlach, J. (1989): A
qualitative theory of the screening-binding effects of magnesium
salts on epithelial cell membrane: a new hypothesis. Magnes.
Res. 2, 243-247.
24. Theophanides, T., Angiboust, J.F..Anastassopoulou. J.
& Manfait. M. (1990): Possible role of water structure In
biological magnesium systems. Magnes. Res.
3, 5-13.
All articles by Dr. Durlach are copyrighted, and permission is
granted to Web users only to make single hard copies for personal
use. Additional reprints should be obtained from the originating
journals. Excerpts may be used by the media with attribution to
Dr. Durlach.
This page was first uploaded to The Magnesium Web Site on
April 15, 1996
http://www.mgwater.com/






