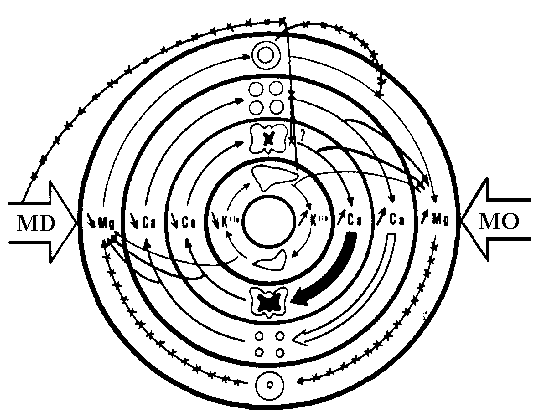
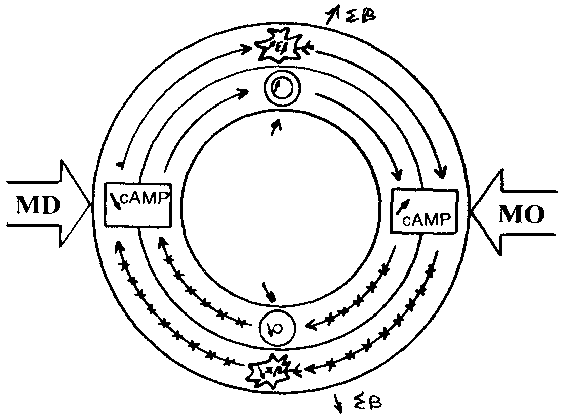
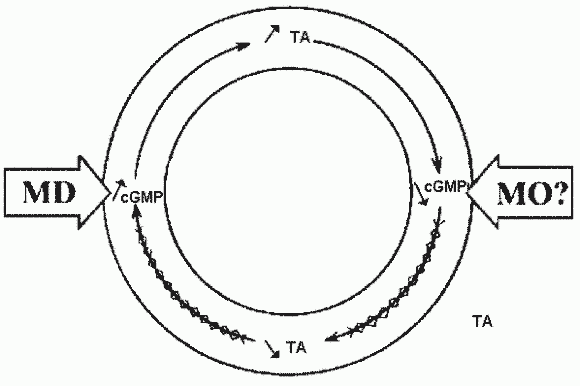
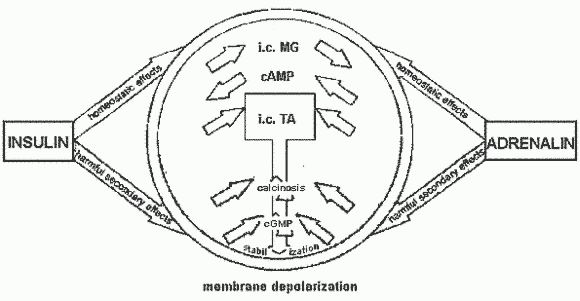
The Magnesium
Online Library
Center for Magnesium Education & Research, LLC
http://www.magnesiumeducation.comMagnesium Symposium at Experimental Biology 2010
Program Announcement, April 24, 2010, Anaheim Convention CenterFeatured Editorial from Life Extension Magazine, Sept. 2005:
Complete Book by
Dr. Mildred S. Seelig:
Free ebook
edited by Robert Vink and Mihai Nechifor
University of Adelaide Press
2011
John Libbey Eurotext
The legal battle for recognition of the importance of dietary magnesium:
Healthy Water Association
THE MAGNESIUM
ONLINE LIBRARY
Ray Tackaberry, Successor Librarian
P.O. Box 1417
Patterson, CA 95363