In: Magnesium in Health and Disease. Y. Itokawa and J. Durlach
eds. John Libbey. London, 1989; 173-182
Magnesium level in drinking water: its importance in
cardiovascular risk
J. Durlach*, M. Bara**, A. Guiet-Bara**
*CHU Cochin--Port Royal, Pt SDRM, 2 Rue de Villersexel
F-75007 Paris, France; **P.M. Curie, Paris, France
INTRODUCTION
Of all the cardiovascular risk factors, magnesium now takes
first place as judged by the accumulation of epidemiological,
pathophysiological, clinical and experimental data, both
pharmacological and therapeutic. The "water story" began in 1957
when, after observing a geographical correlation between
stroke-associated mortality and river water acidity Kobayashi
inferred a possible relationship between the composition of
drinking water and cardiovascular diseases. Schroeder checked the
validity of the Japanese data statistically and then examined the
implications of the phenomenon in the USA. He suggested an
inverse relationship between various types of heart diseases and
drinking water hardness: drinking soft water increases
cardiovascular risk and this effect is reciprocally reduced by
hard water consumption 23-26. Numerous studies in all
the continents have been devoted to this negative correlation
with hardness, involving many statistical units of observation
(states, districts, towns), diversification of causes of
mortality (general, cardiac, vascular) and multiplications of
observed variables (climatic and geographical factors, analytical
data on water). This inverse relationship has been confirmed in
the majority of the studies and in particular in those done on
the largest geographical scale. However, the variable relating to
water hardness cannot be considered a constant risk since in some
studies its effect may be completely absent, the statistical
significance of the other ecological variables involved in the
water story should not be underestimated 23-26,34, and
other confounding aetiological and atmospheric co-factors (ie
amount of rainfall, salt-consumption) which are associated with
hardness may play a role. However the most important aim must be
to define clearly the factors in drinking water which may be
involved in maintaining the cardiovascular apparatus in
satisfactory condition. It is therefore necessary to have an
accurate definition of hardness. Hardness is defined by
hydrotimetry, ie a measurement which determines the presence of
encrusting properties which antagonize vegetable cooking and soap
lathering. The bulk of total hardness is made up by
Ca and Mg compounds but in some areas salts of other metals are
also present in significant amounts : Al, Ba, Fe, Mn, Sr.
Carbonate (or
temporary) hardness denotes the proportion of hardness
chemically equivalent to the concentration of
carbonate-bicarbonate and is thus an index of water alkalinity
and buffer capacity. Non carbonate (or permanent)
hardness denotes the proportion of hardness attributable
to anions such as sulphate or chloride 9,20,41,54. The
variables involved in hardness are of three types: (1) Several
metals, but mainly Ca and Mg determining total hardness; (2) The
buffer capacity and alkalinity of CO3H-CO3
in carbonate hardness; (3) In non carbonate hardness, some of the
numerous factors involved in the aggressive and mainly corrosive
properties of the water, such as SO4-2 and
Cl- 20,41. The beneficial and deleterious
effects of the multiple macro and trace cations and anions
concerned in the measurement of water hardness should be assessed
first on the basis of their own properties, then through their
reciprocal interactions with Mg 23-26,54. These water
factors only need to be taken into account when they represent
quantitatively a significant part of the Recommended Dietary
Allowances or if, qualitatively, they constitute a higher
bioavailable intake.
I - PROTECTIVE FACTORS (PF) IN DRINKING WATER
I.1 - Accessory PF in drinking water
A significant inverse correlation has been irregularly found
between cardiovascular mortality and natural water elements: Ca,
K, As, Cu, Fe, Li, Ni, vaNa-dyl, Zn, CO3H, Cr, I, F,
Se, Si, Zn 23-26,71,48. It is obvious that if the
importance of dietary Ca and K is critical, the quantitative part
of water Ca and K is rarely substantial
23-26,28,30,38,39,43,51,64, 66,73,75,79. The Ca level
in drinking water has importance in advance of the consumer,
since it acts as a crucial anticorrosive substrate of the
protective coating deposited on the pipes by well balanced water
25,26. The inverse correlation between Li and
mortality is low and reverses when the partial correlation is
calculated. Besides, the lowered carbonate and non carbonate
hardnesses are essential components of corrosivity
25,26,54. The water contribution of various trace
elements, the minimum requirements of which are known (F, I, Cr,
Fe, Zn, Cu) may be important 18,25,26,37,48, but it is
difficult to appreciate this contribution when the requirements
have not yet been defined.
I.2 - Mg: prominent PF
Among all the factors studied in drinking water, the highest
inverse correlation has been observed between Mg and
cardiovascular mortality and morbidity. The myocardial Mg level
is significantly lower in soft water areas than in hard water
areas. The same is found in the heart of the subjects who have
died from sudden heart attacks with or without myocardial
infarcts (even outside possibly necrosed tissues where the Mg
leakage depends on tissue lysis). The strongest correlation
concerns fatal arrhythmic seizures
15,23-26,34,50,53,54. The importance of the Mg intake
in drinking water is both quantitative and qualitative.
I.2.1 - Quantitative importance
Water Mg may represent the amount of Mg required to bring an
insufficient dietary Mg level to a correct level
23-26,54. In fact, the adult Mg intake is marginal in
developed countries 23-26,35,47,69 and in some
cardiovascular disease populations particularly
38,43,51,64,66,73,75. According to the variability of
the water intake (tap water, bottled waters, commercialized
beverages using tap water in their preparations), and with a
consumption ranging from less than 1 litre to 2 litres/day with
the corresponding Mg content varying from less than 5 mg to more
than 100 mg/litre, the water Mg intake may quantitatively
represent the critical contribution allowing the direct control
of a marginal intake 3,25,26, 54. Indirectly, the Mg
level of cooking water also intervenes in the Mg intake. There is
an inverse correlation between the Mg loss in the cooked food and
the Mg level of the cooking water: the Mg loss in cooked food is
lower when the food is cooked in a water with a high Mg content.
Nowadays housewives often use hot softened water for cooking: it
would be better to use unsoftened tap water 23-26,60.
Furthermore the concept of nutrient density confers special
importance on water Mg in the Mg intake of the diet. Usually, Mg
intake and calory intake are closely related and the Mg
nutritional density of Mg in most foods rich in Mg is low. Mg in
drinking water constitutes a Mg intake whose high nutritional
density is ideal since it is ... free from calories!
4,31.
I.2.2 - Qualitative importance
Several metals and Mg particularly, ingested in waterborne
solution are more readily assimilated by the intestinal tract
than the same quantity in food 19. A supplementation
study on baboons (Papio Ursinus) shows that tap water
was more effective in ensuring Mg (or Zn requirements) than
dietary supplementation 68. This advantage does not
come from a simultaneous water intake, hydration being an
efficient means to increase urinary Mg leakage 21. It
necessarily results from a higher bioavailability: either
increased absorption (better biodisposability) and/or increased
utilization, probably linked to the biological significance of
the hexahydrated form of Mg 6. Analysis of
pharmacological data in normal human beings of LOWIK and
BINNERTS, of epidemiological and experimental data of NOVIKOW,
and of our own data on membrane permeability 6 allows
us to suggest an explanation of the prominent role of Mg in water
as a cardiovascular protective factor. The presence of highly
bio-available Mg in the intake would avoid the possibility that
the neuroendocrine control of Mg homeostasis might be activated
by a deficient intake of highly bio-available magnesium, ie a
"qualitative" Magnesium deficit, even in the presence of a
satisfactory overall Mg intake. Since the various regulatory
mechanisms involved in Mg homeostasis are the same as those
controlling vasomotor tone and the metabolism of water, Cl, Na,
K, P and Ca, it may be presumed that their dysfunction might
induce cardiovascular ailments 23-26.
Consequently water Mg intake may intervene in cardiovascular
pathology through its quantitative or qualitative properties. The
critical quantitative intake of water Mg which palliates
marginal Mg deficiency may explain its importance as a protective
cardiovascular factor in the case of "absolute" Mg deficit. Mg
deficit may induce numerous deleterious effects either directly
on the nephro-cardiovascular apparatus or indirectly through the
neuro-endocrine apparatus 23-26, but it would be
erroneous to attribute to Mg deficit a major role as a
hypertensive factor. The studies which attribute an
antihypertensive effect to a physiological intake of Mg have
confused pharmacological and physiological data 13,
have disregarded in vivo data showing that Mg deficiency
usually induces hypotension or normotension 1, or have
not taken into account the fact that plasma magnesium is
generally normal in hypertensive individuals 30 and
that normotension is usually the rule during primary Mg deficit
23,24. It seems very important to discredit the myth
that Mg is a major antihypertensive nutrient because of its
potentially harmful consequences for health. For example
epidemiological studies are necessarily interpreted on the
grounds of well established pathophysiological bases. Therefore,
as Mg is erroneously considered as a major antihypertensive
nutrient, it is presented in the Honolulu heart study as ranking
first among other multiple dietary factors showing inverse
association with blood pressure. The same epidemiological data
could be differently interpreted in the light of our background
knowledge of the relationship between Mg and blood pressure
8,17,23,24,27, 29,32,44,49,59,61,67 and by using the
interesting concept of multicollinearity associated with the data
concerning the relation between blood pressure and Ca, P, K and
particularly Na-sensitivity 38,64. Though
Mg does not appear as a major antihypertensive nutrient, Mg
deficit through its numerous noxious actions, both directly on
the nephro-cardiovascular apparatus and indirectly through the
neuroendocrine apparatus, may sometimes, behave as a cofactor of
a hypertensive factor in particular cases. Therefore it is
important to prescribe palliative treatment for the deleterious
effects on the nephro-cardiovascular apparatus of spontaneous or
iatrogenic Mg deficit observed in cardiovascular pathology
23-26,30,38,45, 67.
This quantitative mode of action of water Mg proves useful by
supplying an amount of Mg which allows one to obtain a balanced
daily Mg intake palliating an "absolute" Mg deficit.
A supply of water Mg may also protect the neprocardiovascular
apparatus because of its qualitative mode of action.
Even in the case of quantitatively sufficient daily Mg intake,
water Mg, through its high degree of bioavailability can reduce
the activation of the neuroendocrinoregulatory mechanisms of Mg
homeostasis by a genuine "relative" Mg deficit due to
a deficient intake of the highly bioavailable Mg of water Mg.
This beneficial effect may arise from the role of these
neuroendocrine mechanisms in the control of the metabolism of
water, Cl, Na, K, P and Ca and vasomotor tone (Fig. 1, General
diagram of the endocrine feedback control of humoral disorders
due to alterations in magnesium metabolism.).
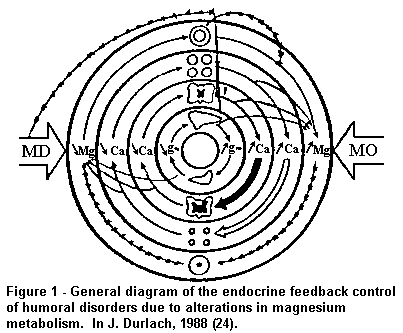
"Absolute" and "relative" magnesium deficit (MD) induces
increased secretion of several neurohormones which are pathogenic
for the cardiovascular system, ie adrenaline, insulin and PTH,
and it also induces a decrease in secretion of CT, a hormone with
protective properties for the cardiovascular system. In addition,
MD induces other neurohormonal or metabolic disturbances which
may have deleterious consequences, for example increased
production of renin and aldosterone, calcinosis, dyslipidaemias
and alterations in haemostasis. An adequate intake of water Mg
may in particular palliate "absolute" marginal MD with a critical
supply over the marginal intake, and/or qualitatively palliate a
"relative" MD by supplying Mg of high bio-availability.
II - NOXIOUS FACTORS (NF) IN DRINKING WATER
II.1 - Natural NF
A significant and direct correlation has been irregularly
found between cardiovascular risk and Na 23-26,28,42.
However this correlation is not consistent 30,42,43,64
and even inverse correlation may be observed 11,51.
These data agree with the notion of salt-sensitive hypertension
with hyporeninaemia and salt-insensitve or salt-resistant
hypertension with normo-or hyper-reninaemia. These clinical forms
of essential hypertension present with abnormalities of plasma
water and electrolytes and variations of plasma volume which are
closely linked with disturbances of calcium-phosphorus regulating
hormones 10,11,16,42,52,56,58,65,66. Chloride, the
anionic component of salt, seems to play an important role,
together with sodium, in the harmful effects involved in
salt-sensitive hypertension. This fact calls into question the
innocuous nature of chlorination processes in water treatment
22,46,76-78,80. It is obvious that the amount of Na
and Cl in drinking water is not a major part of their intake in
the diet, but in salt-sensitive hypertension it is difficult to
reduce the 50% contribution to the total sodium intake which is
provided by meat and dairy products. It is worthwhile, however,
avoiding the consumption of artificially softened water or
mineral waters rich in sodium. A direct but irregular correlation
has also been found between cardiovascular morbidity and other
natural elements of the mineral content of drinking water (Ca, P,
Zn) 25,26,30, but usually these deleterious effects
have been observed in association with markers of dysregulation
of metabolisms: ↑Ca2+ and ↓Ca total in
blood, hypercalciuria, hypophosphataemia and alterations of
calcium and phosphorus controlling hormones 12,
16,23,24,30,39,42,51,58,62,63,66,79. This notion of the
dysregulation of these controlling hormones explains how
sometimes a treatment which reduces the phosphate load of the
arterial wall may be useful in hypertension 62,63; and
how sometimes phosphate therapy may be used as a palliative of
hypophosphataemia, which is inversely related to blood pressure.
Phosphorus reduces hypercalciuria and therefore increases the
level of Ca2+ in the blood, probably through a
reduction in synthesis of 1-25 (OH)2 D3 23,24.
Evidently water intake represents only a small part of the
overall intake of these natural noxious factors.
II.2 - Polluting "factors" and RYZNAR Index
- In the "water story" an important role is played by
corrosivity. This physical factor of water solubilizes toxic
metals in the geological layers or in the pipes
25,26,54. One should distinguish between aggressivity:
the capacity of water to attack calcium carbonate, well defined
in the Langelier Index (IL less than 0 = aggressive water) and
corrosivity: its ability to corrode metals, as defined by the
Ryznar Index (IR greater than 7 = corrosive water). We have
proposed that the RYZNAR Index should be systematically used in
epidemiological studies on the relationship between drinking
water and health 25,26.
- Some polluting metals or metalloids have toxic effects which
may concern the nephro-cardiovascular apparatus: As, Co, Cr, F,
Mg, Mn, Zn, V5- 2,14,25,26,55,70.
- The noxiousness of corrosive waters is mainly due to two
toxic metals, Pb and Cd, which have cumulative toxicity.
In areas where drinking water is corrosive, the main origin of
chronic lead poisoning is water lead pollution 7,23-26,36,
40. Water pollution is a less important aetiological factor
in chronic Cd poisoning, which is more often correlated with
tobacco smoking 23-26,72. Even low levels of Cd and Pb
have detrimental cumulative effects on the cardiovascular system
25, 26. Mg is a competitive inhibitor of Pb and Cd in
the isolated amnion, but on different sites 5,25. Mg
deficiency increases while Mg load decreases toxicity of these
two polluting metals. The target of this antagonism seems to be
cellular since neither absorption nor urinary leakage are
concerned in it 23-26,33, 57,59,72,74.
Mg does not appear to be a panacea which antagonizes all
noxious agents, but it seems to be an antagonist of the two main
polluting metals which induce the nephro-cardiovasotoxicity of
some corrosive drinking waters.
III - NEW PERSONAL DATA
Our studies on the permeability of the isolated human amnion,
estimated by the measurement of ionic conductance and ionic flux
25 complete the preceding data and are summed up in
the following two tables (MS = maternal side; FS = fetal side)
:
| |
MS |
FS |
| Mg Cl2 |
S - B |
S - B |
| Mg SO4 |
S |
B |
| Mg Lactate |
S |
S - B |
| Mg Acetate |
S - B |
S - B |
| Mg Citrate |
S - B |
S - B |
| Mg Nitrate |
S |
B |
Table 1: Effects of the magnesium salts on
the amniotic membrane expressed in terms of screening (S:
reduction of the permeability) and binding (B: increase of the
permeability).
These results show first that water Mg exhibits better tissue
bioavailability probably linked to the hexahydrated form of Mg
and, secondly, that the action of Mg in water is not due to its
hydrated structure only, but also, to its relationship with the
anion present in the medium 6.
| |
MS |
FS |
| Mg - Pb |
na |
CI |
| Mg - Cd |
CI |
na |
| Mg - Hg |
nCI |
nCI |
| Mg - Al |
A |
A |
| Mg - Co |
na |
na |
| Mg - As |
na |
na |
| Mg - Cr |
na |
na |
| Mg - Nn |
Ac |
Ac |
Table 2: - Relationship between Mg and
noxious cations on the external membrane sites obtained after the
measurement of the variation of the conductance (na = no action;
nCI = non competitive inhibition; CI = competitive inhibition; A
= activation with independent fixation; Ac = activation on the
same site, coupling activation).
Mg does not antagonize all noxious agents but reverses the
effects of the two main polluting metals, Pb and Cd 5
which are responsible for the renal and cardiovascular toxicity
of some corrosive drinking waters. Since Pb and Cd are involved
at different sites, Mg may have a common action on both, though
it is more active against Pb than against Cd.
The fact that the amnion is a particularly leaky and
non-excitable membrane limits the scope of our conclusions about
the general properties of membranes. However in spite of the
heterogeneity of the effects, some conclusions can be drawn: (1)
The effect of water Mg may be due to the hexahydrated form in
connection with the linked anion; (2) Water Mg may antagonize Pb
and Cd on the plasma membrane, particularly during combined
intoxication.
CONCLUSION
- Among the numerous variables involved in the "water story"
Mg appears preeminant. Its importance is both quantitative and
qualitative. The critical quantitative intake of water Mg may
palliate an "absolute"
Mg deficit and its multiple consequences particularly on
the nephrocardiovascular apparatus. Even in the case of a
balanced daily Mg intake, water Mg may qualitatively act on the
nephrocardiovascular apparatus by palliating a genuine
"qualitative" Mg
deficit due to a deficient amount of highly bioavailable
Mg. It reduces the activation of the neuroendocrine regulatory
mechanisms of Mg homeostasis which also control the metabolisms
of water, Cl, Na, K, P and Ca and the regulation of vasomotor
tone.
- Corrosivity is the other main factor of the "water story". Mg
appears as a competitive inhibitor of the two main noxious
polluting agents: Pb and Cd.
- It is advisable to have 30 mg/litre of Mg in drinking water. If
the water is not corrosive, it is not advisable to enrich it with
Mg in the course of the processing since its corrosivity index
would also increase. A Mg salt can only be added after the water
has been collected. If the water is corrosive, it will be
filtered in processing stations through an anticorrosive filter
with optimum Mg/Ca ratio 25,26 to ensure the highest
Mg/Ca ratio in tap water with the best anticorrosive power.
References
1. Altura, B.T., Altura, B.M. (1987): Cardiovascular actions
of magnesium: Importance in etiology and treatment of high blood
pressure. Magnesium-Bull 9, 6-21.
2. Anasuya, A., Narasinga Rao, B. S. (1983): Effect of F, Si
and Mg on the mineralizing capacity of an inorganic medium and
stone formers urine tested by a modified in vitro
method. Biochem. Med. 30, 146-156.
3. Antoine, J.M., Magliola, C., Couzy, F., Darret, G.,
Mareschi, J.P. (1986): Estimation of the share of each water
source for adults in France. Water intake provided to the French
adults. Ann. Nutr. Metab. 30,
407-414.
4. Astier-Dumas, M., Gargominy, N., Hoint, F. (1984): Densite
nutrionnelle en magnesium: apropos de quelques produits prets a
etre consommes. Med. Nutr. 20,
397-399.
5. Bara, M., Guiet-Bara, A., Durlach, J. (1987): Toxic metals
and human amniotic ion permeability. I. Effects on the total
transamniotic transfer. Trace Elements in Medicine
4, 154-159.
6. Bara, M., Guiet-Bara, A., Durlach, J. (1988): Analysis of
magnesium membranous effects: binding and screening.
Magnesium Research 1, 29-34.
7. Bonnefoy, X., Huel, G., Guegen, R. (1985): Variation de la
plombemie en fonction de la contamination par le plomb de l'eau
livree a la consommation. Water Res.
19, 1299-1303.
8. Boston, J.L., Beauchene, R.E., Cruikshank, D.P. (1987):
Prospective study of erythrocyte magnesium in teenage pregnancy:
relationship to blood pressure and pregnancy induced
hypertension. J. Am. Coll. Nutr. 6,
41.
9. Bremond, R., Perrodon, C. (1979): Parametres de la qualite
des eaux, 2nd ed. pp. 261, Paris-Neuilly: Ministere de
l'Environnement et du Cadre de Vie.
10. Breslau, N.A., McGuire, J.L., Zerwekh, J.E., Pak, C.Y.C.
(1982): The role of dietary sodium on renal excretion and
intestinal absorption of calcium and on vitamin D metabolism.
J. Clin. Endocrinol. Metab. 55,
369-373.
11. Brinton, G.S., Jubiz, W., Lagerquist, L.D. (1975):
Hypertension in primary hyperparathyroidism: the role of the
renin-antiotensin system. J. Clin. Endocrinol. Metab.
41, 1025-1029.
12. Campese, V.M., Saglikes, Y., Iseki, K., Massry, S.G.
(1984): The relationship between body phosphate and blood
pressure. Adv. Exp. Med. Biol. 178,
271-274.
13. Charbon, G.A. (1986): Effects of magnesium on central
hemodynamics and central circulation. Magnesium-Bull
8, 230-236.
14. Chavassieux, P., Boivin, G., Chapuy, M.C., Meunier, P.J.
(1986): La fluorose osseause a l'eau de Saint-Yorre. Concours
Medical 108, 2387-2391.
15. Chorazy, W. (1986): Magnesiumhaltiges Grubenwasser als
Naturelle dieses Spurenelementes. Magnesium-Bull
8, 114-116.
16. Cirillo, M., Siani, A., Nunziata, V. et al.
(1983): Riddoto rapporto Mg/Ca nelle urine e rischio di
nefrolitiasi nell'ipertensione essenziale. Minerva
Nefrologica 30, 213-216.
17. Cohen, L. (1988): Magnesium and hypertension.
Magnesium-Bull 8, 93-94.
18. Darret, G., Couzy, F., Antoine, J.M. et al.
(1986): Estimation of minerals and trace elements provided by
beverages for the adult in France. Ann. Nutr. Metab.
30, 335-344.
19. Dawson, E.B., Frey, M.J., Moore, T.D., McGanity, W.J.
(1978): Relationship of metal metabolism to vascular disease
mortality rates in Texas. Am. J. Clin. Nutr.
31, 1188-1197.
20. Degremont, G. (1978): Mamento technique de l'eau.
8th edn. pp. 1202 Paris: Technique et Documentation.
21. Delabroise, A.M., Charransol-Maistre, Legrand, KS. et
al. (1984): Etude du comportement de certains parametres
biologiques chez les hommes normaux au cours d'une cure de
diurese. Med. Nutr. 20, 329-355.
22. Douglas, B.H., Revis, N.W., McCauley, P.T., Bull, R.J.
(1983): Chlorinated drinking water increases plasma cholesterol.
Fed. Proc. 42, 871.
23. Durlach, J. (1985): Le magnesium en pratique clinique, ed
J.B. Baillere EMI, pp. 412 Paris.
24. Durlach, J. (1988): Magnesium in clinical practice, Publ.
John Libbey, pp. 386 London-Paris.
25. Durlach, J., Bara, M., Guiet-Bara, A. (1984): Taux de Mg
de l'eau de boisson et facteur de risque cardiovasculaire.
Coimbra Medica 51, 129-142.
26. Durlach, J., Bara, M., Guiet-Bara, A. (1985): Magnesium
level in drinking water and cardiovascular risk factor: a
hypothesis. Magnesium 4, 5-15.
27. Gold, M.E., Buga, G.M., Byrns, R.E. et al.
(1988): Extra-cellular Mg depletion enhances vascular EDRF and
endothelium dependent cyclic GMP formation whereas Mg repletion
causes endothelium and cyclic GMP-independent relaxation.
FASEB. J. 2, A710.
28. Gruchow, H.W., Sobocinski, K.A., Barboriak, J.J. (1985):
Alcohol, nutrient intake and hypertension in US adults.
J.A.M.A. 253, 1567-1570.
29. Gunther, T., Ising, H., Babisch, W., Vormann, J. (1984):
Magnesium intake and blood pressure of spontaneously hypertensive
rats. Magnesium-Bull 6, 120-123.
30. Harlan, W.R., Hull, A.L., Schmouder, R.L., et al.
(1984): Blood pressure and nutrition in adults: the National
Health and Nutrition Examination Survey. Am. J.
Epidemiol. 120, 17-28.
31. Harper, A.E. (1987): Evolution of recommended dietary
allowances: new directions? Ann. Rev. Nutr.
7, 509-537.
32. Henderson, D.G. (1986): Effect of magnesium
supplementation on blood pressure and electrolyte concentrations
in hypertensive patients receiving long term diuretic treatment.
Br. Med. J. 293, 664-665.
33. Hermann, J., Lonchamp, P., Duc, M. (1985): Metabolisme du
magnesium au cours du saturnisme chronique: influence du
traitement chelateur. Magnesium 4,
312-315.
34. Hewitt, D. (1988): Magnesium content of drinking water:
statistical and public health aspects. Trace Subst. Environm.
Hlth. 22 (sous presse).
35. Hill, A.D., Morris, E.R., Ellis, R. et al.
(1986): Mineral balance of adult men consuming self-selected
diets. Fed. Proc. 45, 375.
36. Huel, G., Boudene, C., Jouan, M., Lazar, P. (1986):
Assessment of exposure to lead of the general population in the
French community through biological monitoring. Int. Arch.
Occup. Environ. Health. 58, 131-139.
37. Januszko, T., Karczewski, Suszczynska-Falecka, Z.,
Rybaczuk, M. (1981): Czestose wystepowania wola u dzieci
zamieszkalych na wsi a stezenie jodu, wapmia i magnezu wodach do
picia na terenie woj. bialostockiego. Roczn. PZH
32, 37-43.
38. Joffres, M.R., Reed, D.M., Yano, K (1987): Relationship of
magnesium intake and other dietary factors to blood pressure: the
Honolulu heart study. Am. J. Clin. Nutr.
45, 469-475.
39. Jones, M.R., Martins, J.E., Clemens, R.A. (1988): Mineral
balance and blood pressure in the young spontaneously
hypertensive rat. J. Nutr. 118,
114-120.
40. Kaminski, P., Leone, J., Duc, M. (1988): Incidence du
saturnisme hydrique dans un service de medecine interne en region
de sols acides. Presse Medicale 17,
419-422.
41. Kemmer, F.N. (1979): The NALCO water book, Maindenhead:
MacGraw-Hill, pp. 799.
42. Kesteloot, H. (1984): Epidemiological studies on the
relationship etween Na, K, Ca and Mg and arterial blood pressure.
J. Cardiovasc. Pharmacol. 6/Suppt., S182-S197.
43. Khan, K.T., Barrett-Connor, E. (1987): Dietary potassium
and stroke-associated mortality. N. Engl. J. Med.
316, 235-240.
44. Ku, D.D., Hyung, S.A. (1987): Magnesium deficiency
produces endothelium-dependent vaso-relaxation in canine coronary
arteries. Pharmacol. Exp. Therap. 241,
961-966.
45. Kuller, L., Farrier, N., Caggiula, A. et al.
(1985): Relationship of diuretic therapy and serum magnesium
levels among participants in multiple risk factor intervention
trial. Am. J. Epidemiol. 122,
1045-1057.
46. Kurtz, T.W., Al-Bander, H., Morris, R.C. (1987):
"Salt-sensitive" essential hypertension in men: Is the sodium ion
alone important? N.Engl.J.Med. 317,
1043-1048.
47. Lakshmanan, F.L., Rao, R.B., Kim, W.W., Kelsay, J.L.
(1984): Magnesium intakes, balances and blood levels of adult
consuming self-selected diets. Am. J. Clin. Nutr.
40, 1380-1389.
48. Lanzola, E., Turconi, G., Allegrini, M. et al.
(1983): Relation between coronary heart disease and certain
elements in water and diet. Prog. Biochem. Pharmacol.
19, 80-88.
49. Lau, K., Oasa, C. (1984): Interactions between Mg and
blood pressure. Adv. Exp. Med. Biol.
178, 275-290.
50. Leary, W.P. (1986): Content of magnesium in drinking water
and deaths from ischemic heart disease in white South-Africans.
Magnesium 5, 150-153.
51. McCarron, D.A., Morris, C.D., Henry, H.H., Stanton, J.L.
(1984): Blood pressure and nutrient intake in the U.S.
Science, 224, 1392-1398.
52. McCarron, D.A., Lucas, P.A., Schneidman, K.J. et
al. (1985): Blood pressure development of the spontaneously
hypertensive rat after concurrent manipulations of dietary
Ca2+ and Na+. Relation to Ca2+
fluxes. J. Clin. Invest. 76,
1147-1154.
53. Marier, J.R., Neri, L.C. (1985): Quantifying the role of
magnesium in the interrelationship between human
mortality/morbidity and water hardness. Magnesium
4, 53-59.
54. Marier, J.R. (1986): Role of magnesium in the "hard water
story". Magnesium-Bull 8, 194-198.
55. Marier, J.R. (1986): Interrelations between magnesium and
Co or Al. Magnesium-Bull 8,
293-296.
56. Mayfield, R.K., Bell, N.K. (1985): No evidence for
regulation of plasma renin by 1,25-dihydroxyvitamin D3. N.
Engl. J. Med. 312, 1195-1196.
57. Miller, R.G., Greathouse, D., Bull, R.J., Doerger, J.U.
(1985): Absorption of lead from drinking water with varying
mineral content. In Adv. Modern Environ. Toxicol. eds
E.J. Calabrese, R.W. Tuthill, L. Condie, pp. 289-297 Princeton
Scientific Publ. Co.
58. Morris, C.D., Resnick, L., Sealey, J., McCarron, D.A.
(1987): Is renin predictive of ionized calcium or blood pressure
response to calcium supplementation? Kidney Inter.
31, 304.
59. Nishiyama, S., Saito, N., Konishi, Y. (1987): The
mechanisms of lowered blood pressure in magnesium deficient rats
and magnesium deficient rats fed cadmium. J. Jap. Soc.
Magnesium Res. 6, 73-80.
60. Oh, G.K., Lucker, P.W., Wetzelsberger, N., Kuhlmann, F.
(1986): The determination of Mg, Ca, Na and K in assorted foods
with special attention to the loss of electrolytes after various
forms of food preparations. Magnesium-Bull.
8, 297-302.
61. Ohlhaberry, J., Reyes, A.J., Acosta-Barrius, T.N. et al.
(1987): Pilot evaluation of the putative antihypertensive effect
of magnesium. Magnesium-Bull. 9,
181-184.
62. Plante, G.E., Lafreniere, M.C., Pham Thi Tam, Sirois, P.
(1988): Effect of indapamide on phosphate metabolism and vascular
reactivity. Am. J. Med. 84/sup. 1B,
26-30.
63. Plante, G.E., Dessurault, D.L. (1988): Hypertension in
elderly patients. A comparative study between indapamide and
hydrochlorothiazide. Am. J. Med. 83/sup.
1B, 98-103.
64. Reed, D., McGee, D., Yano, K., Hankin, J. (1985): Diet,
blood pressure and multicollinearity. Hypertension
7, 405-410.
65. Resnick, L. (1987): Interrelations of calcium and
magnesium with Renin-Sodium factors in essential hypertension.
J. Am. Coll. Nutr. 6, 62-63.
66. Resnick, L.M. (1987): Uniformity and diversity of calcium
metabolism in hypertension. A conceptual framework. Am. J.
Med. 82/sup. 1B, 16-26.
67. Reyes, A.J. (1985): Deleterious effects of
antihypertensive treatment on magnesium turnover. Progr.
Pharmacol. 6/1, 51-87.
68. Robbins, D.J., Sly, M.R., Du Bruyn, D.B. (1981): Serum
zinc and demineralized water. Am. J.Clin. Nutr.
34, 962-963.
69. Rouaud, C., Berthier, A.M., Galan, P. (1986): Consommation
alimentaire d'etudiantes de la region parisienne: etude
particuliere du Mg. Med. Nutr. 22,
295-300.
70. Scheuhammer, A.M., Cherian, M.G. (1983): The influence of
manganese on the distribution of essential trace elements II. The
tissue distribution of Mn, Mg, Zn, Fe and Cu in rats after
chronic Mn exposure. J. Toxicol. Environ. Health
12, 361-370.
71. Seurre, C., Varet, F. (1986): Fluor et prevention.
Information Dietetique 2, 42-48.
72. Smetana, R.H., Glogar, D.H. (1986): Role of Cd and Mg in
pathogenesis of idiopathic dilated cardiomyopathy. Am. J.
Cardiol. 57, 364-366.
73. Sowers, M.F.R., Wallace, R.B., Lemke, J.H. (1985): The
association of intakes of vitamin D and calcium with blood
pressure among women. Am. J. Clin. Nutr.
42, 135-142.
74. Victery, W., Miller, C.R., Goyer, R.A. (1986): Essential
trace metal excretion from rats with lead exposure and during
chelation therapy. J. Lab. Clin. Med.
107, 129-137.
75. Weinsier, R.L., Norris, D. (1985): Recent developments in
the etiology and treatment of hypertension: dietary Ca, fat and
magnesium. Am. J. Clin. Nutr. 42,
1331-1338.
76. Whitescarver, S.A., Holtzclaw, B.J., Downs, J.H. et al.
(1986): Effect of dietary chloride on salt-sensitive and
renin-dependent hypertension. Hypertension
8, 56-61.
77. Wones, R.G., Glueck, C.J. (1986): Effects of chlorinated
drinking water on human lipid metabolism. Environ. Health
Persp. 69, 255-258.
78. Wyss, J.M., Liumsiricharoen, M., Spiraiorojthikoon, W.
et al. (1987): Exacerbation of hypertension by high
chloride, moderate sodium diet in the salt-sensitive hypertensive
rat. Hypertension 9/sup. III, III
171-III 175.
79. Young, E.W., Bukoski, R.D., McCarron, D.A. (1988): Calcium
metabolism in experimental hypertension. Proc. Soc. Exp.
Biol. Med. 187, 133-141.
80. Zicha, J., Kunes, J., Jelinek, J., Leonteva, G.R.,
Govyriu, V.A. (1986): The importance of sodium and chloride ions
for the development of DOCA-NaCl hypertension: a haemodynamic
study. Physiol. Bohemoscov. 35,
308-312.
All articles by Dr. Durlach are copyrighted, and permission is
granted to Web users only to make single hard copies for personal
use. Additional reprints should be obtained from the originating
journals. Excerpts may be used by the media with attribution to
Dr. Durlach.
This page was first uploaded to The Magnesium Web Site on
December 24, 1995
http://www.mgwater.com/

