JOURNAL OF APPLIED NUTRITION, VOLUME 34, NUMBER 2, 1982
GUEST EDITORIAL
THE CALCIUM CONTROVERSY
Guy E. Abraham, M.D.*
It is often stated that large amounts of calcium are required
for strong bones, to calm nerves and for other characteristics of
good health. Some nutritionists recommend up to three grams of
calcium a day to prevent calcium deficiency. The purpose of this
editorial is to review some aspects of Human Evolution,
Physiology, Biochemistry and Dietary Habits in order to clarify
calcium requirements and its close relationship to intake of
other nutrients, mainly magnesium.
EVOLUTIONARY CONSIDERATIONS
Over the past 6000 years or more man evolved in a magnesium
and potassium-rich, but calcium and sodium-poor, environment. For
survival, the human body had to develop efficient conserving
mechanisms for sodium and calcium. To conserve sodium, the Zona
Glomerulosa of the Adrenal Cortex secretes a very potent
mineralocorticoid, Aldosterone, which increases sodium retention
via the kidney 27. To conserve calcium, the skin
developed a synthetic process that manufactures Vitamin D3 from a
cholesterol derivative, under the influence of solar ultraviolet
radiation. Vitamin D3 is then hydroxylated by the liver to
25-OH-D3. The kidney is the site of the most important step:
1-hydroxylation of 25-OH-D3 to generate 1, 25 (OH)2 D3, the most
potent calcium-conserving substance16. It increases
calcium and phosphate absorption in the small intestine and
decreases calcium excretion in the urine:
PHYSIOLOGICAL CONSIDERATIONS
The 1-hydroxylase is located in the kidney as a mitochondrial
enzyme. It is sensitive to intramitochondrial calcium and
phosphate. Intromitochondrial accumulation of both calcium and
phosphate depress the activity of 1-hydroxylase, thereby
decreasing formation of 1, 25 (OH)2 D322.A low
phosphate diet increases and a high phosphate diet depresses 1,
25 (OH)2 D3 production20.
Besides 1, 25 (OH)2 D3, there are two hormones that play an
important role in calcium metabolism: Calcitonin (CT) and
Parathyroid Hormone (PTH)3. Both hormones are
sensitive to serum ionized calcium levels. An increase in serum
ionized calcium results in stimulation of CT secretion and
suppression of PTH secretion.
CT and PTH regulate skeletal turnover of calcium and
availability of cytoplasmic calcium3. The major
skeletal effect of PTH is to increase bone resorption by
stimulating osteoclasts, thereby increasing mobilization of
calcium from bone. PTH also favors cellular uptake of calcium by
soft tissues and phosphate excretion by the kidney. CT has the
opposite effect, that is, it increases deposition of calcium in
the bone matrix and blocks cellular uptake of calcium by soft
tissues. Magnesium suppresses PTH and stimulates CT
secretion28, therefore favoring deposition of calcium
in the bone and removal of calcium from soft tissues. Furthermore
magnesium enhances calcium absorption and retention5,
12, whereas increasing calcium intake suppresses magnesium
absorption2, 25.
BIOCHEMICAL CONSIDERATIONS
Calcium and magnesium are often antagonistic in their effect
of biological reactions7. For example, the
biosynthesis of both phospholipids and proteins involve enzymatic
steps which have an obligatory requirement for magnesium and are
calcium-inhibited. The glycolytic pathway contains five enzymatic
reactions that have an absolute requirement for magnesium and
require optimal magnesium/calcium ratio for peak performance.
In order for the cell to maintain the proper magnesium/calcium
ratio, several levels of regulation are available, acting on the
removal of calcium from the cytoplasm. One such mechanism is the
ATP-dependant calcium pump in the cell membrane 9, 10.
The other important mechanism is the transport of calcium inside
the mitochondria. The mitochondria uptake of calcium is
reversible if calcium concentrations in the microenvironment are
kept below certain limits. Above these limits, calcification of
mitochondria occurs with subsequent cellular death. In the
presence of magnesium, the uptake of calcium by mitochondria can
be slowed down. Since ATP utilization is magnesium-dependent, it
becomes obvious that the calcium pump at the cell membrane is
also magnesium-dependent. The generation of ATP itself through
the glycolytic pathway is in part magnesium-dependent and
inhibited by calcium.
DIETARY CONSIDERATIONS
Stable civilizations have arisen only when primitive hunting
communities have learned to cultivate cereals, such as wheat,
rice maize, millets, barley, oats and rye. In many rural areas,
cereals provide more than 70% of the energy consumed9.
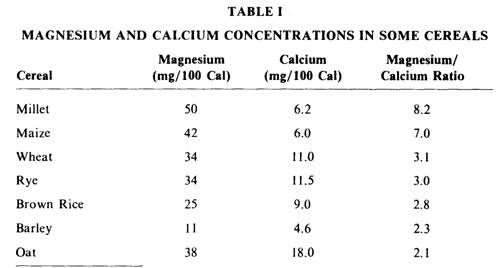
Table I shows the magnesium and calcium concentrations in these
staple foods. They contain two to eight times more magnesium than
calcium, and as much as one thousand milligrams of magnesium
could be consumed if two thousand calories were obtained from
these sources. One may argue that dairy products contributed to
most of the ingested calcium. This is unlikely since 50% of
individuals tested so far show allergic reactions to dairy
products and lactose intolerance is common in most ethnic groups,
occurring in 70% of Black Americans and over 70% of Orientals,
Jews, Arabs, Greeks, Japanese, Eskimos, Indians, Africans and
Asians 23, 17, 13, 14, 15, 1, 24, 18, 8, 19 ,30,
31.
Considering that 99% of the total body calcium is located in
the bones, it is not surprising that academic proponents of high
calcium intake have used as an argument the possible role of
calcium deficiency in osteoporosis 11, 4, 29. There is
no evidence, however, to support this view. Osteoporosis is not
more common in those parts of Asia and Africa where diets are
relatively low in calcium (300-500 mg/day) than in Europe and
North America where consumption of dairy products contributes to
more than1000 mg of calcium/day When patients with severe
osteoporosis were given massive doses of calcium they went into
positive calcium balance, but radiographic studies revealed no
changes in the osteoporotic process Where did that calcium go?
Obviously into the soft tissues where it does not belong.
Calcium balance studies have indicated that man can adapt to
relatively low calcium intake by increasing calcium absorption
and decreasing urinary excretion10. There is not such
a mechanism for magnesium26. The adaptation to low
calcium intake is most likely via synthesis of 1, 25 (OH)2 D3 by
the kidney. It was previously discussed that high
intramitochondrial concentrations of phosphate and calcium in the
kidney suppress the formation of 1, 25 (OH)2 D3 20,
22. Therefore, mechanisms that increase intracellular and
intramitochondrial calcium would prevent adaptation to low
calcium intake. Failure of the calcium-pump at the cell membrane
and increased uptake of calcium by mitochondria are two such
mechanisms which are both magnesium-dependent as previously
discussed. Since a low phosphate diet increases formation of 1,
25 (OH)2 D3 20 and a high magnesium diet would keep
calcium out of the mitochondria, it seems therefore that one
approach to improving the adaptation to low calcium intake is to
ingest a diet low in phosphate and high in magnesium. Such an
approach to the management of osteoporosis would seem more
appropriate than the ingestion of massive doses of calcium. The
latter approach blocks magnesium absorption and creates a
magnesium deficiency, conducive to a failure of the calcium- pump
and intracellular accumulation of calcium in soft tissues that
eventually leads to irreversible cell damage. Also, magnesium
deficiency results in elevated PTH which prevents the utilization
of the absorbed calcium for bone formation and favors soft tissue
calcification.
Recent studies suggest that calcium requirements are increased
by acid-ash, high- protein and high sulfur diet21. In
order to increase the efficiency of the adaptation mechanism to
low calcium intake, every attempt should be made to ingest foods
containing a magnesium/calcium ratio of two or more, with neutral
or alkaline ash, not excessive in phosphate, sulfur, proteins,
refined sugar, fats and other substances that drain the body of
both calcium and magnesium. Magnesium deficiency causes a reduced
intestinal absorption of calcium and decreased serum ionized
calcium.
Magnesium has a calcium-sparing effect and decreases the need
for calcium.
Since magnesium suppresses PTH and increases CT, adequate
magnesium intake would improve the phosphorous balance from a low
phosphate diet by increasing phosphate absorption via the 1, 25
(OH)2 D3mechanisms and by preventing the PTH induced
phosphaturia. Furthermore, a high magnesium intake would enhance
calcium absorption by the 1, 25 (OH)2 D3mechanisms, increase
serum ionized calcium, promote deposition of calcium in the bone
matrix where it belongs and minimize cellular uptake and
mitochondrial accumulation of calcium. )
With such an approach there would be no need for
pharmaceutical companies to develop new and improved calcium
blockers in the management of cardiovascular diseases, since
magnesium works naturally to produce the same end result.
REFERENCES
1. Alzante, H. Gonzalez, H. and Guzman, J. “Lactose
intolerance in South American Indians.” Am. J. Clin.
Nutr. 22: 122, (1969).
2. Amiot, D., Hioco, D. and Durlach, J. “Frequence du
deficit magnesique chez le sujet et dans diverses
osteopathies.” J. Med. Besancon 5:371-378,
(1969).
3. Aurbach, GD., Marx, S.J. and Spiegel, AM.
”Parathyroid Hormone, Calcitonin, and Calciferols.”
In textbook of Endocrinology, Williams, RH. (Ed),
Saunders Co., 922-1032, (1981).
4. Aviolo, LV. “Postmenopausal osteoporosis: prevention
versus cure.” Fed. Proc. 40: 2418, (1981).
5. Briscoe, A.M. and Ragen, C. “Relation of magnesium on
calcium metabolism in man.” Am. J. Clin. Nutr. 19:
296-306, (1966).
6. Bryan, W.T.K. and Bryan, M.P. ”Cytotoxic Reactions in
the Diagnosis of Food Allergy.” Otol. N. Am. 4:
523-533, (1971).
7. Bygrave, F.L. “Cellular Calcium and Magnesium
Metabolism.” In An Introduction to Bio-inorganic
Chemistry. Williams, D. R. (Ed) Thomas, 171-184, (1976).
8. Cook. G.C. and Kajubi, SK. “Tribal incidence of
lactase deficiency in Uganda.” Lancet l: 725,
(1966).
9. Davidson, S., Passmore. R., Brock, J.F. and Truswell, AS.
“Human Nutrition and Dietetics.” Churchill
Livingstone, 166-175, (1979).
10. Davidson, S., Passmore, R., Brock, J.F. and Truswell, A.S.
“Human Nutrition and Dietetics.” Churchill
Livingstone, 90-106. (1979).
11. Draper, H.H. and Scythes, C.A. ”Calcium,
phosphorous, and osteoporosis.” Fe. Proc. 40:
2434, (1984).
12. DuRuisseau, J.P. and Marineau, J.M. “Osteoporose
medication calcique et magnesienne,” See Int’l
Sympos on Magnesium, 223-226, (1971/1973).
13. Gilat, T., et. al. “Lactase deficiency in Jewish
communities in Israel.” Am J. Digest. Dis. 16:203,
(1971).
14. Gilat. T., et. al “Lactose intolerance in an Arab
population.” Am. J. Digest. Dis. 16:203,
(1977)
15. Gudmand-hoyer, and F., Jarnum, S. “Lactose
malabsorption in Greenland Eskimos.” Acta Med.
Scand. 186:235, (1969).
16. Holick, M.F. and Clark, MB. “The photobiogenesis and
metabolism of Vitamin D.” Fed. Proc. 37:
2567-2574, (1978).
17. Huang, S.S. and Bayless, T.M. “Milk and lactose
intolerance in healthy orientals.” Science 160:
83, (1968).
18. Johnson, J.D., et. al. “Lactose malabsorption among
the Pima Indians of Arizona.” Gastroenterology 73:
985, (1977).
19. Kretchmer, N., et.al. “Intestinal absorption of
lactose in Nigerian ethnic groups.” Lancet 2: 392,
(l971).
20. Larkins, R.G., McAuley, S.J., Colston, K.W., Evans,
I.M.A., Galante, L.S. and Macintyre, I. “Regulation of
Vitamin D. Metabolism without Parathyroid Hormone.”
Lancet: 289-291, (1973).
21. Linkswiler, H.M., Zemel, M.B., Hegsted, M., and Schuette,
S. “Protein-induced hypercalciuria.” Fed.
Proc. 40:2429, (1981).
22. MacIntyre, I. “Vitamin D and the integration of
Calcium Regulating Hormones.” In First European
Symposium on hormones and Cell Regulation. Dumont, J. and
Nunez. J. (Ed) North Holland, 195-208, (1977).
23. Nasrallah, SM. “Lactose intolerance in the Lebanese
population and in ‘Mediterranean lymphoma’.”
Am. J. Clin. Nutr. 32:1994-1996, (1979).
24. Newcomer, AD., et. al. “Family studies of lactose
deficiency in the American Indian.”
Gastroenterology 73; 1299, (1977).
25. Parlier. R., Hioco, D. and LeBlanc, R. “Les troubles
du metacolisme magnesien. Symptomes et traitment des carences et
des plethores magnesiennes.” Rev. Franc. Endocr.
Clin. 4: 335-339, (1963).
26. Rude, R.K., Bethune, J.E. and Singer, F.R. “Renal
tubular maximum for magnesium in normal, hyperparathyroid and
hypoparathyroid man.” J. Clin. Endocrinol. Metab.
51: 1425-1431, (1980).
27. Schrier, R.W. and Leaf, A. “Effect of Hormones on
Water, Sodium, Chloride, and Potassium Metabolism.” In
Textbook of Endocrinology, Williams RH. (Ed) Saunders
Co., 1032-
28. Seelig, MS. “Magnesium Deficiency in the
Pathogenesis of Disease.” Plenum Medical Book
Company, 3 17-321, (1980).
29. Seeman, E. and Riggs, B.L. “Dietary prevention of
bone loss in the elderly.” Geriatrics 36:71-79,
(1981).
30. Senewiratne, B., et. al. “Intestinal lactase
deficiency in Ceylon (Sri Lanka).”
Gastroenterology 72:1257, (1977).
31. Shibuya, S. et. al. “Lactose intolerance in Japanese
children.” Advan. Med (Japan).
72:323, (1970).
*Optimox Inc.
801 Deep Valley Dr.
Rolling Hills Estates, CA 90274
Published and distributed by
International College of Applied Nutrition
Box 386. La Habra California 90631
ISSN No. 0021-8960
This page was first uploaded to The Magnesium Web Site on July
20, 2002
http://www.mgwater.com/

