| Epidemiologic Reviews |
Vol. 19, No. 2 |
| Copyright © 1997 by The Johns Hopkins
University School of Hygiene and Public Health |
Printed in U.S.A. |
| All rights reserved |
|
Desalinated seawater Articles
Magnesium in Drinking Water and Ischemic Heart Disease
Arthur Marx and Raymond R. Neutra
INTRODUCTION
Ischemic heart disease (IHD), codes 410-414 and 429.2 in
the International Classification of Diseases, Ninth Revision,
Clinical Modification (ICD-9-CM) (1), is the leading cause
of mortality in many industrialized countries. Among people over
age 45 years in the United States, IHD claims more lives per year
than any other cause of death. Data from the Third National
Health and Nutrition Examination Survey (1988- 1991) indicate
that an estimated 11.2 million Americans live with IHD (2). In
1992, the most recent year for which mortality data have been
published, 480,170 people died of IHD (2). As many as 300,000
sudden cardiac deaths, or even more, occur in the United States
each year (3). Because of this heavy population burden of
mortality, a factor that prevented even a small proportion of
these deaths would save thousands of lives each year and have
major public health significance.
In the 1950s, '60s, and '70s, epidemiologists were intrigued
when some but not all ecologic studies suggested that "hard"
(alkaline) water might have a protective effect against IHD. Many
of the waters sampled may have been high in magnesium.
Subsequently, in the 1970s, '80s, and '90s, studies have seemed
to indicate a beneficial effect of magnesium itself. The
preponderance of these population studies of drinking-water
magnesium have found odds ratios above 1.0, despite the fact that
they must have been carried out with considerable nondifferential
exposure misclassification and that the dosage of magnesium
derived from drinking water was very small in comparison with
usual dietary intake. Besides studies linking magnesium with the
development of IHD in the general population--the first stage of
pathogenesis--the epidemiologic evidence relates magnesium to
three additional points in the natural history of IHD: 1) the
point after the presence of cardiac risk factors has been
established; 2) the immediate aftermath of a myocardial
infarction; and 3) the years following a first myocardial
infarction. This paper reviews the strengths and weaknesses of
the epidemiologic evidence relating magnesium to the three stages
of the natural history of IHD. We also review the substantial
biologic evidence suggesting that magnesium could affect smooth
muscle and cardiac muscle irritability, impulse transmission,
atherosclerosis, and other physiologic processes which are
disrupted in persons with IHD. Finally, a number of possible
research options are presented.
MAGNESIUM BALANCE
The human body contains approximately 20,000 mg of magnesium,
of which 60 percent (12,000 mg) is found in bone and 20 percent
(4,000 mg) in skeletal muscle; the rest circulates in the serum
(200 mg) or is divided among other tissues (3,800 mg) (4). The
serum level is maintained between 17 mg/liter and 26.7 mg/ liter.
Approximately 50-65 percent of serum magnesium is in the ionized
form (4). With a dietary intake of about 300 mg/day, 200 mg would
pass through the body in the feces, and 100 mg would be absorbed
into the serum and then excreted in the urine. These estimates do
not account for increased loss caused by physical stress and
sweating (5). Thus, a typical daily absorption of 100 mg of
magnesium is equivalent to 1/38th of the amount stored in tissues
other than bone and skeletal muscle. Although it is true that if
dietary intake falls dramatically, the kidney is capable of
reabsorbing the vast majority of magnesium from the glomerular
filtrate, producing urine with almost no magnesium content, a few
months of marginally deficient intake could considerably deplete
some body compartments of their magnesium (4).
MAGNESIUM TOXICITY
In the absence of renal failure, the kidney is capable of
excreting large amounts of absorbed or injected magnesium ion so
rapidly that serum levels usually do not become dangerously high
(6). The high doses used clinically rarely cause a degree of
hypermagnesemia likely to be associated with serious side
effects, because the patients are closely monitored. Potentially
toxic and even lethal effects can occur when magnesium-containing
drugs, usually antacids or cathartics, are ingested chronically
by individuals with renal insufficiency (7, 8). The toxic effects
of elevated serum magnesium progress in severity with increasing
concentration and include nausea, vomiting, hypotension,
bradycardia, urinary retention, electrocardiographic changes,
hyporeflexia, central nervous system depression, and, at higher
concentrations, life-threatening respiratory depression, coma,
and asystolic cardiac arrest (9). A daily intake of several
hundred additional milligrams of magnesium from water or
supplements can cause diarrhea and abdominal cramps in
susceptible individuals (6). After high doses of mostly
parenteral application of magnesium, a sharp drop in blood
pressure, hyporeflexia, and respiratory paralysis due to central
nervous system depression may occur (10, 11). At the most severe
level of hypermagnesemia, respiratory depression, coma, and
asystolic cardiac arrest may result (9). Clinical experience and
the literature show that magnesium has a large therapeutic range.
The various randomized trials of postmyocardial infarction
magnesium cited below used thousands of milligrams of magnesium
per day with no untoward effect. Magnesium hydroxide (milk of
magnesia) is an over-the-counter laxative whose prescribed oral
dose is 700-1,600 mg/day; thus, a laxative effect is achieved
only at doses two orders of magnitude higher than the doses of
waterborne magnesium dealt with in the studies reviewed here.
WATERBORNE VERSUS FOODBORNE MAGNESIUM
In the general population, the major portion of magnesium
intake is via food and, to a lesser extent, water (12). Balance
studies reviewed by Seelig led her to the conclusion that an
intake of at least 6 mg/kg per day is needed to ensure adequate
magnesium status (13). The Recommended Dietary Allowance (RDA) of
4.5 mg/kg per day is based on the upper range of requirements
determined in recent metabolic balance studies (14). Surveys of
the food intakes of individuals have revealed that a majority of
the US population consumes less than the RDA of both calcium and
magnesium; many subjects took less than 80 percent of the RDA for
magnesium (8, 15, 16).
Waterborne magnesium has been suggested as a supplemental
source of highly bioavailable magnesium (17-20). Two experimental
pilot studies (17, 21) using urinary analyses revealed that
waterborne magnesium is absorbed about 30, percent better and
faster than dietary magnesium. Animal supplementation studies
showed that tap water was more effective in meeting magnesium
requirements than dietary supplementation (22). It is possible,
then, that waterborne magnesium could correct an insufficient
dietary magnesium level (23). Most of the studies discussed below
dealt with waterborne magnesium at a level of about 10 percent of
total daily magnesium intake. We will discuss below the issue of
whether the apparent effects of waterborne magnesium are
compatible with the above dietary facts.
DOCUMENTED BIOLOGIC EFFECTS OF MAGNESIUM
Magnesium is a cofactor in more than 300 enzyme systems in
human cells, and it has a pivotal position in normal myocardial
physiology. Possible sites of action include vascular smooth
muscle (24-29), platelets (30-32), and myocardial cells. Animal
experimental studies (33, 34) and biochemical laboratory studies
(35) show that magnesium is an essential cofactor for
sodium-potassium adenosine triphosphatase, an enzyme that
influences cardiac irritability by regulating the concentration
gradient of sodium and potassium across myocardial cell
membranes. The influence of magnesium on the electron transport
system is essential for transmembrane ion flux,
excitation-contraction coupling, energy metabolism, and
prevention of early atherosclerotic changes (36). Magnesium
deficiency may be involved in the initiation and propagation of
free radical myocardial tissue damage through oxidation of
myoglobin, which is essential for intracellular transport and
storage of oxygen (37-39). Magnesium helps to suppress
arrhythmias during ischemia and reperfusion (40-42). Animals fed
on a magnesium-deficient diet developed larger infarcts than did
control animals (43). At pharmacologic doses, magnesium is
thought to mimic and to be an antagonist of calcium, i.e., a
natural calcium channel blocker, possibly because of its
structural similarity as a divalent cation (44). Magnesium
deficiency in rats causes hyperlipidemia and subsequently
atherogenic depositions in coronary arteries, and is involved in
the development of endothelial lesions preceding atherosclerosis
(45). Arsenian (46) provides a comprehensive review of the
available evidence.
CLINICAL TRIALS OF MAGNESIUM AFTER MYOCARDIAL INFARCTION
Very high doses of up to 2,200 mg of intravenous magnesium
have been proposed to improve survival immediately after
myocardial infarction. It is not clear how relevant any findings
from such high doses would be to the prevention of IHD by lower
doses. Not all 16 randomized trials that included magnesium as a
treatment option showed a statistically significant benefit.
Meta-analyses (47-49) were performed on data from 10 randomized
clinical trials in which the clinical use of magnesium in the
treatment of arrhythmias, myocardial inflections, transient
ischemic attacks, and hypertension was investigated among 3,900
patients with suspected acute myocardial infarction. Individuals
treated with intravenous magnesium postinfarction were at
significantly lower risk of dying from IHD-related complications.
A review (50) of another five small randomized, controlled trials
found that two of them showed a statistically significant
reduction in total mortality, and the other three found a
non-significant trend in the same direction. The Fourth
International Study of Infarct Survival (ISIS-4) (51), a large
randomized, controlled multicenter trial which showed no
beneficial effect on infarct survival or IHD-related
complications, failed to produce conclusive results. The use of
fibrinolytic therapy postmyocardial infarction before
randomization appears to be the most criticized design feature of
ISTS-4 (52-54). There has been one randomized trial (55) of 365
mg of magnesium given orally on an ongoing basis to ambulatory
patients who had already had a first myocardial infarction.
Surprisingly, the magnesium group showed a statistically
significant increase in subsequent myocardial infarctions. If
confirmed, this would raise questions about supplementing the
water supply of an entire population, which would include
postmyocardial infarction patients.
MAGNESIUM AFTER THE PRESENCE OF CARDIAC RISK FACTORS HAS BEEN
ESTABLISHED
Singh (56) carried out a prospective 10-year dietary
intervention study in India in which 400 urbanized individuals
with prevalent coronary risk factors volunteered to follow either
a magnesium-rich diet or their usual diet. A daily magnesium
intake of up to 1,400 mg was reached in the intervention group by
diet modification without supplements. Intake of waterborne
magnesium was not taken into account There were significantly
fewer IHD-related complications in the intervention group (p <
0.001), as well as a lower incidence of sudden cardiac death, IHD
mortality, and total mortality (p < 0.01). Lower fat and
cholesterol intake may, among other factors, have confounded the
association between magnesium intake and IHD morbidity. It seems
likely that Singh observed the effect of various nutritional
variables, as affected by the 10-year intervention.
AUTOPSY STUDIES OF MYOCARDIAL MAGNESIUM CONCENTRATIONS
Sharrett (57) and Eisenberg (58) have provided detailed
discussions of relevant autopsy studies. Table 1 shows results
from studies that specifically dealt with myocardial magnesium
concentrations. In none of the studies found in the literature
(59-65) did investigators succeed in controlling for the fact
that postmortem low myocardial magnesium concentrations may have
been a consequence rather than a cause of cardiac death. The
studies found that 1) decedents who died from IHD had
significantly lower magnesium levels in heart muscle and
diaphragm muscle than did decedents who died from accidents and
2) substantially more controls from soft water areas had low
magnesium concentrations in coronary arteries than controls from
hard water areas. In none of these studies were the statistics
adjusted for age at death or stage of IHD. Choosing persons who
died via accidents as the control group may have introduced
confounding bias, because such deaths are often alcohol-related.
In addition, chronic alcohol consumption is known to be related
to magnesium deficits, but this would have biased differences
toward zero (66).
STUDIES OF WATER HARDNESS AND THE POPULATION INCIDENCE OF
CARDIOVASCULAR DISEASE
In 1957, Kobayashi (67) first suggested an inverse relation
between vascular disease and the acidity of drinking water in
Japan; stroke-related death rates appeared to be higher in soft
water (higher acidity) areas than in hard water areas. Studies
conducted before the 1980s were primarily focused on levels of
water hardness. The relevance of these studies to the magnesium
hypothesis must be viewed with caution. Extensive reviews by
Punsar (68), Neri et al. (69), Comstock (70), and Sharrett (57)
failed to find conclusive relations between calcium and magnesium
in water and heart disease. Table 2 and Figure 1 provide an
overview of correlational studies which relate the calcium and
magnesium content of drinking water to cardiovascular disease
mortality (69, 71-88).
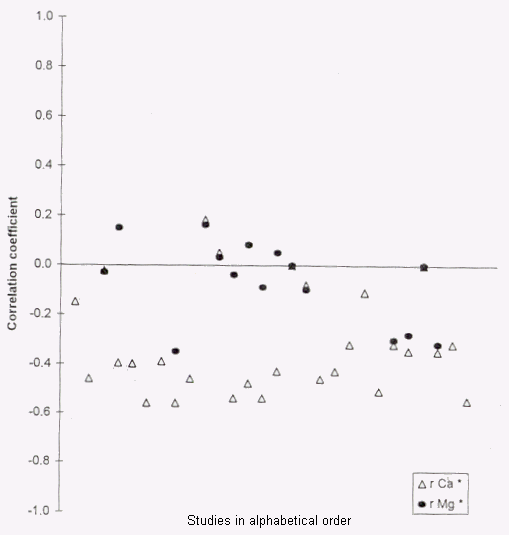
FIGURE 1. Correlations between water hardness and
cardiovascular mortality in various studies. Δ, calcium
(Ca);  , magnesium (Mg). *r,
Pearson's poduct-moment correlation coefficient. Coefficients
shown pertain to different study methods, age/sex groups, and
ICD-9-CM codes (see table 2).
, magnesium (Mg). *r,
Pearson's poduct-moment correlation coefficient. Coefficients
shown pertain to different study methods, age/sex groups, and
ICD-9-CM codes (see table 2).
ECOLOGIC STUDIES OF MAGNESIUM IN DRINKING WATER AND IHD
Correlation-based ecologic studies
We found 12 ecologic studies which reported correlation
coefficients between rates of IHD (or IHD death) and the
magnesium content of local waters (72, 76, 78-80, 84, 85, 87,
89-92). These studies showed contradictory results.
Correlation-based ecologic studies may not be optimally sensitive
and may not be specific enough to differentiate between a
spurious relation and a true relation of water minerals with IHD
morbidity and mortality (93). Cardiovascular deaths represent a
complex interaction in which lifestyle factors play a large role.
Some studies carried out in the United States (84, 85, 94) and in
England and Wales (79) showed an inverse correlation between
death rates from hypertensive and arteriosclerotic heart disease
and calcium and magnesium levels in drinking water. Other
investigations in the United States (78) and Canada (90) showed
inverse but statistically nonsignificant correlations between
magnesium concentrations in water and IHD mortality.
Two Swedish (72, 80) studies demonstrated a relation between
water hardness and mortality from arteriosclerotic heart disease,
but no relation to magnesium. Both studies had important
shortfalls: The average values rather than the mineral levels of
individual waterworks were analyzed; magnesium concentrations
were measured years after the disease study period; and areas in
which the water composition had changed were not excluded. A
cross-sectional study conducted in South Wales which dealt with
tap water obtained at home rather than water at the nearest
treatment plant (76) failed to demonstrate a significantly
inverse correlation between calcium and magnesium and mortality
from coronary heart disease and stroke. Shaper et al. (86) found
a significant relation between cardiovascular mortality and water
hardness in Great Britain, but no association with magnesium.
Adjustment for climatic and socioeconomic factors reduced the
magnitude of the water hardness effect. The study apparently did
not distinguish between IHD and stroke. IHD morbidity in US
cities (87) did not correlate significantly with magnesium and
calcium in water when the other element was controlled for by
partial correlation. Karppanen et al. (95, 96) first implicated
the high calcium: magnesium ratio in Finland in that country's
highest IHD and stroke morbidity and mortality among middle-aged
men. Later, Marier and Neri (97) demonstrated a significant
correlation between incidence of IHD and the dietary calcium:
magnesium ratio in various countries.
Rate-based ecologic studies of magnesium and IHD.
Rate-based ecologic studies report actual rates, not just
correlation coefficients. Table 3 summarizes the characteristics
and results of the rate-based studies discussed below. We found
eight studies which presented rates of IHD morbidity or mortality
as a function of the magnesium content of water. Many of these
protective effects resulted from surprisingly low levels of
waterborne magnesium. For most of the studies, information on
confidence limits, significance tests, detailed assessment of
water or nutritional intake, or control for other known risk
factors was not presented. The measurement of exposure in all of
these studies left much to be desired. The amount of tap water
consumed in beverages and in cooking was not ascertained; in
addition, information on the magnesium content of the drinking
water was obtained from official sources at a convenient time
point and sometimes was averaged for areas whose water came from
several different water companies. This would have induced
considerable nondifferential misclassification as to magnesium
intake. Figure 2 shows differences in relative risks and changes
in water magnesium levels in five rate-based ecologic studies
which presented the data in a form that lent them to graphic
presentation.
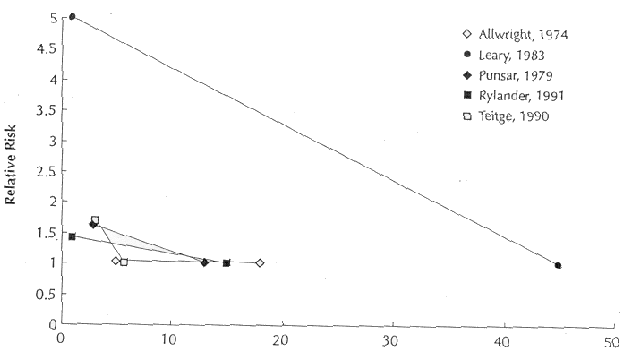
Figure 2. Relative risk of ischemic heart
disease as a function of water magnesium concentration in five
rate-based studies (99, 100, 102, 103, 106).
An early Finnish study on the relation between fluoride and
magnesium concentrations in drinking water focused on the
prevalence of cardiovascular disease (98). Water magnesium
concentrations showed large variability within the monitored
areas. The prevalence of IHD varied markedly and showed no
uniform relation with magnesium water levels.
Allwright et al. (99) matched three Los Angeles, California,
communities supplied by water of different hardness according to
age, sex, race, income, socio-economic status, and stability of
mineral concentrations, using 1970 US Census data. The weighted
mean magnesium contents of the drinking water, which had not
changed in the previous 10 years, paralleled the concentrations
of calcium found in the same areas. When the three areas were,
compared, there was no change in IHD mortality with alterations
in water magnesium and calcium concentrations. Approximately 85
percent of the study population had been exposed to the levels of
magnesium found in municipal drinking water. Migration and
commuting to other areas of Los Angeles could have introduced
misclassification bias as to intake of waterborne magnesium and
calcium. This carefully designed study, carried out in a
circumscribed geographic area with major IHD risk factors
controlled, did not show a significant reduction of IHD morbidity
with change in water magnesium levels.
IHD death rates in South African white males showed a
significant negative association (p < 0.02) with magnesium
levels in drinking water (100, 101). No such association could be
demonstrated in blacks. However, one drawback of the study was
the fact that while mortality was assessed in 1978, water
analyses for the same areas were performed 4 years later.
Teitge (102) conducted a study over a period of 10 years in a
district of East Germany. Myocardial infarction incidence and
water hardness were compared for hard water versus soft water
areas. Magnesium concentrations ranged from 2 mg/liter to 48
mg/liter; the weighted mean magnesium concentrations in five
areas varied from 2.9 mg/liter to 5.8 mg/liter. A 38 percent
decline in myocardial infarction incidence, from 332 per 100,000
population to 206 per 100,000, was found with increasing
concentrations of calcium and magnesium.
Rylander et al. (103) explored water magnesium concentrations
in Sweden and found that changes in the magnesium concentration
at low levels produced significant differences in the incidence
of cardiovascular mortality. Communities with no change in the
chemical composition of their drinking water were included in the
study. Ten-year death rates for IHD and cerebrovascular disease
in 27 municipalities were standardized for age group and related
to mortality data for the entire country, Comparison with the
expected numbers of deaths showed a decrease in the relative risk
from 1.1 for 1 mg/liter to 0.78 for 15 mg/liter.
A recent study conducted in Switzerland (Rylander et al.,
University of Gothenburg (Gothenburg, Sweden), unpublished
manuscript) confirmed the inverse correlation between magnesium
in drinking water and mortality from IHD.
Case-control studies. Luoma et al. (104) conducted a
retrospective hospital- and population-based case-control study
among Finnish males aged 30-64 years, to investigate the effects
of magnesium and fluoride on IHD. Fifty myocardial infarction
patients were pair-matched for age and region (rural vs. urban).
For the population-based case-control pairs, the study showed
significantly elevated risk estimates for low magnesium and
fluoride concentrations. Exclusion of persons under 50 years of
age or persons who had used their present water source for less
than 6 years led to increased risk estimates.
A recent retrospective case-control study of Swedish males
aged 50-69 years by Rubenowitz et al. (12) addressed the relation
between deaths from acute myocardial infarction (ICD-9-CM code
410) between 1982 and 1989 and the level of magnesium in drinking
water. Potentially confounding factors such as sex, age, and
calcium in drinking water were controlled for, but not risk
factors such as diet, exercise, and cholesterol level.
Individuals exposed to higher water magnesium levels, ranging
from <3.5 mg/liter to >9.8 mg/liter, were at significantly
lower risk of death from myocardial infarction. Confounding bias
may have been introduced by selecting males who died of cancer
(ICD-9-CM codes 140-239) as controls, since there are reports in
the literature on an association between magnesium and malignant
neoplasms (105). Although this study offered an increase in
precision regarding exposure to magnesium, the exact magnesium
intakes from water consumed at home or in other places are
unknown, because the amounts of water consumed and the use of
water filters were not documented. There is also uncertainty
regarding the accuracy of diagnosis, which was based on
information obtained from death certificates.
Cohort studies. Punsar and Karvonen (106) conducted a
prospective cohort study in two rural populations of males in
Finland over a 15-year period. Regarding assessment of exposure
to magnesium in drinking water, the study had the characteristics
of an ecologic analysis. In both study areas, drinking water,
collected primarily from wells, was soft. The cohort in eastern
Finland experienced a 1.7 times higher death rate from IHD than
the cohort in the western part of the country. The two
populations seemed to be suitable for a water study, since the
men in the two areas led similar rural lifestyles and magnesium
levels in drinking water were stable. These data support the
hypothesis that magnesium levels in drinking water play a role in
the difference between the IHD mortality rates in the two study
areas.
Four of five ecologic studies (98-100, 102, 103), one cohort
study (106), and two case-control studies (12, 104) showed a
beneficial effect of magnesium.
The relative risks clustered around 1.6, ranging from 1.4 to
7.8. The attributable risks ((relative risk - 1)/ relative risk)
in these studies ranged from 33 percent to 87 percent. The
population attributable risk percentage is not available for
these studies, because their authors did not provide data on the
full distribution of exposures in cases and noncases. Indeed, we
have not found published data on the distribution of a population
according to their source of waterborne magnesium. As a general
observation, it should be noted that few water sources seem to be
rich in magnesium, so calculated population attributable risk
percentages would not be dramatically smaller than attributable
risk percentages.
Dose-response estimates
Assuming a drinking water consumption of 2 liters per day,
estimates of changes in the absolute rates and the relative risks
of cardiovascular morbidity and mortality were obtained (table
4). The decrease in the absolute mortality rates ranged from 0.1
per mg per 100,000 population in the Los Angeles study (99) to
20.0/mg/100,000 in the Finnish study (106). The relative risk
decrease ranged from 0.001 to 0.034 per mg of magnesium. The East
German study (102) showed a decrease in the incidence of
myocardial infarction of 21.7/mg/ 100,000; the relative risk
decreased 0.105 per mg. The Indian food intervention study (56)
showed a decrease in the absolute rates of cardiovascular
morbidity of 1.0/mg/100,000, and a decrease in the relative risk
of 0.002 per mg. As mentioned above, the control group in this
study had a magnesium intake of 300-500 mg/day; for the
intervention group, approximately 700 - 850 mg were added to this
diet, which is already rich in magnesium by Western standards. It
seems possible that an effect could have been observed at smaller
intervention levels. In reviewing the data in table 4, we
assessed whether the absolute or relative effect of magnesium on
IHD was an orderly function of the range between high and low
magnesium water concentrations. For example, does one reach a
point of diminishing returns or, to the contrary, does the effect
become larger as the difference between high and low doses of
waterborne magnesium becomes larger? We saw no orderly
pattern.
RANGE OF APPARENT EFFECTS
How can we explain the seemingly paradoxical finding that
waterborne magnesium, which contributes orders of magnitude less
magnesium to intake than does dietary magnesium, has such a large
apparent effect on cardiovascular health? Calculation of the
proportion of total mortality attributable to magnesium
deficiency would require knowledge of the current range of
intakes. Lacking this information, one could take a hypothetical
city with low levels and, on the basis of results derived from
the above-mentioned studies, calculate the effect of increasing
waterborne magnesium on IHD rates. The calculations suggest that
a small percentage change in total dietary intake, through a
change in drinking water, might produce dramatic changes in IHD
rates. The East German study of IHD incidence by Teitge (102)
provides a number of data points from an ecologic study. It also
provides one of the steepest regression slope estimates. If we
apply this coefficient to a hypothetical city with magnesium
levels at 2.9 mg/liter and increase the magnesium levels to 5.8
mg/liter, the IHD rates would fall from 332/100,000 to 206/
100,000--an absolute drop of 126/100,000, or a 1.6- fold relative
drop. When we express this as a percentage of the higher rate, it
represents approximately a 38 percent reduction (attributable
risk percentage). If, instead, we used the data from Allwright et
al. (99), an absolute and relative decrement of only a few
percent would be achieved. However, even a few percentage points
of difference for a common and serious disease, if real, could be
of public health interest. For example, a few percent (not to
speak of a hypothetical 38 percent) of the 300,000 fatal cardiac
arrests which occur each year in the United States represents
thousands of cases per year.
Since migration may have affected the results of the study by
Allwright et al. (99), we use Teitge's data (102) to illustrate
our point. If one applies Teitge's IHD incidence difference of
126 per 100,000 per year to a city with one million adult
inhabitants, the total burden of avoided IHD each year is 1,260
cases. How reasonable is this? Even assuming that typical persons
drank 2 liters per day of this water with 5.8 mg of magnesium per
liter, they would only be taking in 5.8 mg/day more than they
were when the water contained only 2.9 mg/liter. Water intakes
less than 2 liters/day would contribute even less waterborne
magnesium intake. Investigators such as Marier and Neri (97)
argue that such small amounts of waterborne magnesium are
effective because other dietary sources of magnesium are grossly
deficient. Their arguments are not quantitative, but they seem to
us to be saying that these other sources are so low in magnesium
that even a 5.8 mg/day difference represents a large percentage
supplementation.
Let us assume that the incremental waterborne magnesium needs
to represent the same proportion as the corresponding
proportionate fall of IHD--38 percent in our example. We do not
know what the total dietary East German magnesium intake was at
the time of this study, but we can calculate how low the
foodborne intake would have to be so that increasing the
waterborne intake by 5.8 mg would constitute a 38 percent
increase in total intake. Let X be the foodborne intake. When [(X
+ 11.6) -(X + 5.8)]I(X + 5.8) = 0.38, then X = 9.5
mg/day. That is, the baseline foodborne intake would have to be
as low as 9.5 mg/day for this change of 5.8 mg to constitute a 38
percent increase in total foodborne intake.
Of course, what is important is the absorbed magnesium, not
the ingested magnesium. Let us stipulate that waterborne
magnesium is absorbed 1.3 times more avidly than foodborne
magnesium (17, 21). Assuming 30 percent absorption of foodborne
magnesium and 39 percent absorption of waterborne magnesium (1.3
X 30 percent = 39 percent), it can be shown that a food intake of
27.6 mg of magnesium per day with an increase from 5.8 mg to 11.6
mg of waterborne magnesium daily would correspond to a 38 percent
increase in total absorbed magnesium. This assumes that there is
a linear relation between magnesium intake and IHD risk.
Either of these intakes is very low compared with the 329
mg/day of the typical American male (15) or even the 198 mg/day
mentioned by Altura and Altura (107). This degree of deficiency
seems hard to believe. Such gross deficiency would, one would
think, produce acute symptomatology. Furthermore, similar
associations with magnesium have been seen in South African
whites, Swiss, and Swedes. These are not populations with gross
nutritional deficiencies. It makes more sense to assume that the
East Germans had an intake similar to that of Americans. This, as
we have seen, is somewhere between 198 and 500 mg/day. The added
5.8 mg in this case would thus represent somewhere between 3
percent and 1 percent of the total dietary intake. If people
actually consumed only 1 liter of water per clay, the percentage
would be half as much. Even if we assume that 100 percent of the
waterborne magnesium is absorbed and only 30 percent of the
foodborne magnesium is absorbed, a person ingesting 200 mg of
foodborne magnesium and 11.6 mg of waterborne magnesium daily
would absorb 71 mg of magnesium, only 6 mg more than the total 65
mg of individuals with only 5.6 mg of magnesium intake from
water. This constitutes a 9 percent increase in absorption, which
is not enough to explain a 38 percent change. We have tried
simulating situations in which subgroups of the population are
particularly deficient in magnesium or, in addition to their
deficiency, have an even more striking avidity for waterborne
magnesium. None of these scenarios are compatible with the
production of a 38 percent change in IHD rate. There is another
reason for wondering how such a low percentage increment in total
magnesium absorption could produce observable effects: Other
dietary sources of magnesium may easily vary 10 percent from day
to day and from person to person. It would seem that this small
difference from water would be lost in the noise. In short, if we
require that a given proportional drop in IHD mortality demands a
proportional increase in total magnesium ingestion or absorption,
the qualitative arguments, which allege that dietary deficiencies
in magnesium explain why small differences in waterborne
magnesium can produce moderate benefits in IHD morbidity and
mortality, do not hold up.
Could the paradox be explained by proposing that there is some
dermal absorption during bathing? We found no studies on this
possibility, but it seems unlikely to us. Could it be due to the
bioaccumulation of magnesium in vegetables and animal products in
regions with high magnesium waters? In that case, the water is
merely an indicator of total magnesium intake and cannot be used
to estimate dose-response relationships. This hypothesis requires
that people consume primarily locally grown produce and animal
products. This assumption might be true in parts of Europe, but
certainly not in the United States. It could be tested by
assessing 24-hour urinary excretion in populations in low and
high magnesium water areas to see whether excretion is higher
than that expected from water ingestion alone. The epidemiologic
findings could only be explained by a dose-response curve in
which slight changes in proportional intake produce an
unproportionally large effect. This would mean that a large
proportion of the population was so deficient with regard to
cardiovascular outcomes that the dose-response gradient was very
steep. Under this view, any increase would produce some
improvement until some unknown optimum was reached. Singh (56)
was able to show some apparent benefit by manipulating the total
diet so as to increase magnesium intake by 700 mg/day in persons
whose baseline diet contained 400 mg/day, thus suggesting that
this optimum might be quite high, more than 1,000 mg/day. This
raises the possibility that chronic disease epidemiologists could
provide a substantially different nutritional recommendation than
that which nutritionists would provide on the grounds of
short-term balance studies.
Human nutrient requirements are generally estimated from the
results of depletion and repletion studies, isotopic turnover
studies, and balance experiments (108). The most common method of
estimating human magnesium requirements appears to be the
metabolic balance procedure (109). Balance studies attempt to
determine the dosage of magnesium required before the body begins
eliminating any excess (109-111). When that point is reached, an
adequate intake is assumed. Experts argue about the analytic
methods and populations chosen for such balance studies. The
challenge lies in getting from an average requirement of some
individuals to a dietary recommendation for a population (108).
As of this date, the RDAs (14) have not addressed prevention of
chronic disease (112). RDAs are considered safe and adequate
levels of a nutrient, as part of a normal diet; they apply to
healthy individuals and do not cover nutritional needs arising
from metabolic disorders, chronic diseases, or drug therapies
(14). A variety of host and environmental factors apparently
influence magnesium excretion. Unfortunately, however, the
argument for recommending increases in magnesium intake has had a
physiologic basis. If the apparent discordance between
physiologic and epidemiologic recommendations were to be
sustained, this would have implications for other nutrients as
well. For example, a small subsample of the population might
require more magnesium than others in order to avoid IHD. The
prevalence of this hypothetical group might be low enough that
few or none of them would show up among subjects in a typical
balance study, yet they might account for the majority of the IHD
cases. In our extensive readings, we have not found serious
pharmacokinetic discussions that postulate which body
compartments, rate of exchange parameters, and dose-response
relationships could explain the IHD incidence changes associated
with small differences in waterborne magnesium. Physiologists and
pharmacologists must confer and examine this issue further.
METHODOLOGICAL CRITICISMS
There is really no good way to disprove the possibility that
studies which show an association between magnesium levels in
water or food and IHD risk are more likely to be published.
Studies showing no effect (or no significant effect) of magnesium
have been published as well. We think this is an unlikely
explanation for the results seen.
Another approach to dealing with possible publication bias is
to estimate the number of unpublished negative studies
("fail-safe N") necessary to invalidate the overall
beneficial effect of waterborne magnesium seen in the eight
studies reviewed (113, 114). Meta-analysis of observational
studies has been proposed (Egger and Davey Smith, University of
Berne (Berne, Switzerland), unpublished manuscript), and the
"fail-safe N," as well as "funnel plot" statistics, must
be computed separately for each type of epidemiologic study.
However, information on the rigor of the methods, as well as on
parameters required for this type of analysis, has been provided
in only a few studies (99, 102, 103). The population sizes in
these three studies ranged from 105,000 to 810,000, and the
relative risk estimates for IHD ranged from 1.03 to 1.61, whereas
studies with fewer individuals or a less rigorous study design
produced much higher risk estimates.
Nondifferential misclassification of information on water and
magnesium intake has undoubtedly occurred in these studies, but
it is unlikely to have produced false-positive results,
particularly in eight separate studies with different study
designs. Nondifferential misclassification regarding exposure
would tend to obscure results, so that, if present, the magnesium
effect would be even stronger than that seen in these studies.
Nonetheless, future studies--be they randomized trials for IHD
patients, case-control studies, or ecologic studies--could all do
a better job of quantifying water and mineral intake.
Bias caused by properties of ecologic studies could distort
the exposure-disease association (115). Multiple-group comparison
studies, which describe the association between the average
exposure level and the disease rate in various geographic areas,
are especially prone to this kind of bias. In addition certain
environmental and sociodemographic variables tend to be more
highly correlated with each other on a group level than they are
on the individual level, a phenomenon called muticollinearity
(115, 116). More homogeneous groups or smaller units of analysis
could minimize inferential problems in ecologic studies.
Comstock's review (70) of hard water studies indicated that most
studies conducted within cities or counties showed no
association, or even a reversed association of IHD with water
hardness.
Except for a few studies (12, 75, 86), the possibility of
confounding by other risk factors has not really been addressed
to date. There is no reason to believe that persons in low
magnesium areas would, for instance, smoke more, have a higher
fat intake, exercise less, have more stress, and comprise more
Type A personalities than, those in high magnesium areas.
Nonetheless, future study designs could take potential
confounding into consideration and strengthen the evidence.
In our view, confounding by other waterborne or dietary
factors associated with magnesium is the most serious concern
with all of the studies to date, A full characterization of all
water constituents and their ratios has not been part of the
analysis. Significantly IHD-protective effects were
described--e.g., for fluoride (104) and silicon (85) in drinking
water--and although there seems to be a lack of biologic
plausibility that would explain why such a relation is causal,
these factors could conceivably confound the association between
magnesium and IHD. The Singh study (56) involved changing an
entire diet, not just supplementation with magnesium. Future
studies should carefully document the range of elements and their
chemical forms in the waters being studied. In addition, places
with high levels of magnesium in drinking water might have high
magnesium levels in locally grown food. Analyses of risk should
take all of the constituents into account and should characterize
the particular chemical forms of magnesium being studied. It
would be unfortunate to recommend magnesium supplementation and
then learn that magnesium alone, without consideration of its
ratio to other chemicals, was not helpful and perhaps even
harmful.
FUTURE DIRECTIONS
Our main focus here is on the possibility that magnesium may
play a role in the primary prevention of IHD. This preventive
effect may be expressed through an increase in dietary magnesium
of hundreds of milligrams per day or through much smaller amounts
of magnesium in water or bottled beverages. It could well turn
out that the paradoxical low dose water findings are an anomaly,
while the dietary association might be confirmed. Any serious
study of waterborne magnesium would need to control for dietary
magnesium. The following types of additional studies of the low
dose water findings should be seriously considered.
First, we should review ongoing or completed IHD cohort
studies to determine whether either the waterborne magnesium
hypothesis or the dietary magnesium hypothesis could be evaluated
in them. Ideally, such studies would have obtained dietary and
medical histories and would have patient addresses available so
that local water quality data could be linked to patient records.
For example, available data on magnesium laxative use from large
IHD cohort studies should be reviewed to see whether individuals
exposed to laxatives containing magnesium salts are at lower risk
for IHD.
Second, we should conduct a a large, nested case-control study
of myocardial infarction with and without, sudden death in an
area with naturally occurring high and low levels of magnesium in
the water. Dietary and waterborne intakes could be ascertained in
a random validation sample prospectively; then, as cases
occurred, cases, surrogate relatives, and controls could be
interviewed retrospectively about their diet, vitamin and mineral
intake, water intake, and other cardiac risk factors in the
recent and more remote past. Waters should be sampled for all
minerals and analyzed for their chemical composition. Determining
urinary magnesium and food sample magnesium in a subsample of
cases and controls in high and low waterborne magnesium areas
could address the food bioaccumulation theory mentioned above.
Rylander and colleagues are currently conducting a study with
some of these features in Sweden; this should also be done in the
United States.
Third, the autopsy studies (59-65) suggested that magnesium
levels in the myocardium and diaphragm among subjects who died
accidental deaths were lower when the drinking water level in the
area of residence was low. A confirmation of these findings in a
larger, well-conducted study would increase the credibility of
the waterborne magnesium findings. A study of the magnesium
content of the myocardium, skeletal muscle, bone, and the
arterial wall in accident victims of various ages with no
detectable alcohol in their blood could be conducted in areas
with high and low levels of waterborne magnesium.
Fourth, the ability to benefit from waterborne magnesium
supplementation in the face of overall dietary magnesium
deficiency could only result from an unusually low, genetically
controlled ability to absorb magnesium from food coupled with an
unusually high absorption of magnesium from water. Such persons
might be more prevalent among myocardial infarction survivors
than among age-matched persons without IHD. One could carry out
metabolic balance studies using foodborne and waterborne
magnesium sources to determine whether such people exist and, if
so, their prevalence in IHD and normal populations. It is
essential to understand the dose-response relationships between
magnesium and IHD, both to increase the credibility of the
epidemiologic findings and to guide the recommendations given.
Nutritionists and pharmacologists should conduct theoretical
studies using quantitative pharmacokinetic models to suggest how
it would be possible for one body compartment to become
substantially depleted with a slightly deficient diet.
Additionally, how would it be possible for waterborne magnesium
to have such a marked effect when it constitutes only a few
percent of total intake or absorption? Second, animal studies of
dose-response relationships in the protection against an
atherogenic diet could be carried out. Third, additional studies
of the uptake of foodborne and waterborne magnesium from
different chemical compounds could be conducted. This would also
provide information that could guide medical recommendations
regarding supplement use if it were eventually indicated.
STUDIES OF THE UNTOWARD EFFECTS OF MAGNESIUM
Transient gastrointestinal symptoms
Studies should be conducted to determine the rate of untoward
gastrointestinal symptoms related to various forms and doses of
waterborne magnesium in combination with other minerals, and to
discover whether there is a range of inherent sensitivities. Is
there habituation to high magnesium levels, or is this side
effect permanent? Studies which could address these issues
include short cohort studies of populations, such as college
students or soldiers, that are moving into areas with high and
low levels of magnesium, to determine the incidence and duration
of gastrointestinal symptoms. Gastrointestinal side effects could
be monitored in large-scale clinical trials or in the control
group of any large nested case-control study.
Possible untoward effects of magnesium on other
diseases
Morris et al.'s study (79) examined patterns of death rates
for a variety of causes of death as they related to water
hardness. The overall death rate was lower in hard water areas. A
few causes of death had elevated rates in one sex or the other.
The study reported a correlation of community rates with their
levels of water hardness. This study should be repeated with
better methodology, which could be achieved if the ecologic
studies also considered other causes of death. A case-control
study could examine a sample of non-cardiovascular deaths to see
whether history of magnesium intake was any different from that
of controls in these subjects. This type of study could also be
used to obtain more complete information on subclinical adverse
effects of intermediate to high magnesium intakes, for instance,
undesirable changes in blood levels of other nutrients. Any
large-scale trials could examine the incidence of other diseases
in the treatment groups.
SUMMARY AND CONCLUSIONS
The associations found in the general populations of a number
of different countries are suggestive and warrant an integrated
program of laboratory and epidemiologic research to reject or
confirm the magnesium-IHD hypothesis. Singling out this
particular risk factor has two justifications. First, as would be
the case with any epidemiologic risk factor for IHD whose
attributable risk was large enough to be detectable through
epidemiology, applying that attributable risk to the vast annual
morbidity and mortality from IHD would translate into tens of
thousands of lives benefited and millions of dollars in hospital
costs avoided per year. Second, this particular risk factor could
conceivably be eliminated by an inexpensive supplementation
program. For example, a low-sodium, higher-magnesium and
-potassium table salt has been recommended and used in Finland
for many years, during a period when the prevalence of
hypertension in population surveys was said to decrease (117).
Interventions which do not require behavioral change have always
been the most cost-effective in public health. We therefore urge
funding agencies to give priority to studies determining whether
there are unforeseen, adverse effects of magnesium for some
population subgroups and whether the apparent benefit derived
from low doses of magnesium in the development of IHD or IHD
death is real. Furthermore, researchers should determine which
chemical form of magnesium is best absorbed and most
effective.
We need to better understand the interrelation of various
water and food constituents, as well as individual risk factors,
in the pathogenesis of IHD. Susceptible individuals who are at
higher risk of being depleted of magnesium need to be identified,
and potential untoward effects of magnesium should be studied.
Future research must provide better answers about low level
waterborne magnesium before recommendations to the public can be
made.
ACKNOWLEDGMENTS
Support for this work was provided in part by the Swiss
National Science Foundation (grant 823B-36985). The authors thank
Paul and Janet Mason for drawing their attention to the
magnesium-IHD issue and for providing many relevant articles and
suggestions. The following colleagues provided helpful
suggestions and advice: Drs. Burton Altura, Larry Barrett, Joseph
Brown, George Comstock, Jean Durlach, Mark Eisenberg, Ron Elin,
John Goldsmith, George Kaplan, Carl Keen, David Morry, Mildred
Seelig, Terry Troxell, and Walter Willett.
REFERENCES
1. Commission on Professional and Hospital Activities, US
Health Care Financing Administration. International
classification of diseases, Ninth Revision, clinical
modification. 2nd ed. Washington, DC: US GPO, 1980. (DHHS
publication no. (PHS) 80-1260).
2. American Heart Association. Heart and stroke facts: 1995
statistical supplement. Dallas, TX: American Heart Association,
1995. (Publication no. 55-0520(COM)).
3. Myerburg RC, Castellanos A. Sudden cardiovascular collapse
and death. In: Isselbacher KJ, Braunwald E, eds. Harrison's
principles of internal medicine. 13th ed. New York, NY:
McGraw-Hill, 1994:193-8.
4. Elin RJ. Magnesium metabolism in health and disease. Dis
Mon 1988;34:163-218.
5. Seelig MS. Consequences of magnesium deficiency on the
enhancement of stress reactions: preventive and therapeutic
implications (a review). J Am Coll Nutr 1994;13:429-46.
6. Friedman PA. Poisoning and its management--magnesium. In:
Braunwald E, Isselbacher KJ eds. Harrison's principles of
internal medicine. 13th ed. New York, NY: McGraw-Hill,
1987:846.
7. Lipsitz PJ. Effect of nutrient deficiencies in man. In:
Rechcigl M Jr, ed. CRC handbook series in nutrition and food.
Section E: nutritional disorders. Cleveland, OH: CRC Press,
1978:113-17.
8. Shils ME. Magnesium. In: Shils ME, Olson JA, Shike M, eds.
Modem nutrition in health and disease. 8th ed. Philadelphia, PA:
Lea and Febiger, 1994:164-84.
9. Mordes JP, Wacker WE. Excess magnesium. Pharmacol Rev
1977;29:273-300.
10. Amdur MO, Doull J, Klaassen CD. Magnesium toxicity. In:
Amdur MO, Doull J, Klaassen CD, eds. Casarett and Doull's
toxicology: the basic science of poisons. 4th ed. New York, NY:
Pergamon Press, 1991:668-9.
11. Browning E. Toxicity of industrial metals. 2nd ed. New
York, NY: Appleton-Century-Crofts, 1969.
12. Rubenowitz E, Axelsson G, Rylander R. Magnesium in
drinking water and death from acute myocardial infarction. Am J
Epidemiol 1996;143:456-62.
13. Seelig MS. The requirement of magnesium by the normal
adult. Am J Clin Nutr 1964;14:342-90.
14. National Research Council. Recommended dietary allowances.
10th ed. Washington, DC: National Academy Press, National Academy
of Sciences, 1989.
15. US Department of Agriculture. Nationwide Food Consumption
Survey: Continuing Survey of Food Intakes of Individuals (CSFII).
Hyattsville, MD: Human Nutrition Information Service, US
Department of Agriculture, 1986. (Report 85-1).
16. Alaimo K,,McDowell MA, Briefel RR, et al. Dietary intake
of persons ages 2 months and over in the United States: Third
National Health and Nutrition Examination Survey, phase 1,
1988-91. (Advance data from vital and health statistics, series
14, no. 1). Hyattsville, MD: National Center for Health
Statistics, 1994.
17. Løwik MR, Groot EH, Binnerts WT. Magnesium and
public health: the impact of drinking water. In: Trace substances
in environmental health, XVI: proceedings of the University of
Missouri's 16th Annual Conference on Trace Substances in
Environmental Health. Columbia, MO: University of
Missouri-Columbia, 1982:189-95.
18. Durlach J. The importance of magnesium in water. In:
Durlach J, ed. Magnesium in clinical practice. London, England:
John Libbey and Company Ltd, 1988:221-2, 386.
19. Durlach J, Bara M, Guiet-Bara A. Magnesium level in
drinking water: its importance in cardiovascular risk. In:
Itokawa Y, Durlach J, eds. . Magnesium: in health and disease:
proceedings of the Fifth International Symposium on Magnesium,
Kyoto, Japan, 1988. London, England: John Libbey and Company Ltd,
1988:173-82.
20. Theophanides T, Angiboust J-F Polissiou M, et al. Possible
role of water structure in biological magnesium systems. Magnes
Res 1990;1:5-13.
21. Binnerts WT, Løwik MR, Groot EM. On the importance
of Mg in drinking water. In: Bratter P, Schramel P, eds. Trace
element analytical chemistry in medicine and biology. Vol 2.
Proceedings of the Second International Workshop, Neuherberg,
Federal Republic of Germany, April 1982. New York, NY: W de
Gruyter, 1983:287-93.
22. Robbins DJ, Sly MR, de Bruyn DB. Serum zinc and
demineralized water. (Letter). Am J Clin Nutr 1981;34:962-3.
23. Altura BM, Altura BT. New perspectives on the role of
magnesium in the pathophysiology of the cardiovascular system. I.
Clinical aspects. II. Experimental aspects. Magnesium
1985;4:226-71.
24. Altura BM, Altura BT, Carella A, et al. Hypomagnese6a and
vasoconstriction: possible relationship to etiology of sudden
death ischemic heart disease and hypertensive vascular diseases.
Artery 1981;9:212-31.
25. Hattori K, Saito K, Sano H, et al. Intracellular magnesium
deficiency and effect of oral magnesium on blood pressure and red
cell sodium transport in diuretic-treated hypertensive patients.
Jpn Circ J 1988;52:1249-56.
26. Berthelot A, Esposito J. Effects of dietary magnesium on
the development of hypertension in the spontaneously hypertensive
rat. J Am Coll Nutr 1983;2:343-53.
27. Resnick LM, Gupta RK, Laragh JH. Intracellular free
magnesium in erythrocytes of essential hypertension: relation to
blood pressure and serum divalent cations. Proc Natl Acad Sci U S
A 1984;81:6511-15.
28. Chrysant SG, Ganousis L, Chrysant C. Hemodynamic and
metabolic effects of hypomagnesemia in spontaneously hypertensive
rats. Cardiology 1988;75:81-9.
29. Turlapaty PD, Altura BM. Magnesium deficiency produces
spasms of coronary arteries: relationship to etiology of sudden
death ischemic heart disease. Science 1980;208:198-200.
30. Adams JH, Mitchell JR. The effect of agents which modify
platelet behaviour and of magnesium ions on thrombus formation in
vivo. Thromb Haemost 1979;42:603-10.
31. Watson KV, Moldow CF, Ogburn PL, et al. Magnesium sulfate:
rationale for its use in preeclampsia. Proc Natl Acad Sci U S A
1986;83:1075-8.
32. Rayssiguier Y, Gueux E. Magnesium and lipids in
cardiovascular disease. J Am Coll Nutr 1986;5:507-19.
33. Skou JC, Butler KW, Hansen O. The effect of magnesium,
ATP, P, and sodium on the inhibition of the (Na + K)- activated
enzyme system by g-strophantin. Biochim Biophys Acta
1971;241:443-61.
34. Borchgrevink PC, Oksendal AN, Jynge P. Acute extracellular
magnesium deficiency and myocardial tolerance to ischemia. J Am
Coll Nutr 1987;6:125-30.
35. Garfinkel D, Garfinkel L. Magnesium and regulation of
carbohydrate metabolism at the molecular level. Magnesium
1988;7:249-61,
36. Seelig MS, Heggtveit HA. Magnesium interrelationships in
ischemic heart disease: a review. Am J Clin Nutr 1974;27:
59-79.
37. Freedman AM, Atrakchi AH, Cassidy MM, et al. Magnesium
deficiency-induced cardiomyopathy: protection by vitamin E.
Biochem Biophys Res Commun 1990;170:1102-6.
38. Kramer JH, Misik V, Weglicki WB. Magnesium deficiency
potentiates free radical production associated with post-ischemic
injury to rat hearts: vitamin E affords protection. Free Radic
Biol Med 1994;16:713-23.
39. Weglicki WB, Bloom S, Cassidy MM, et al. Antioxidants and
the cardiomyopathy of Mg deficiency. Am J Cardiovasc Pathol
1992;4:210-15.
40. Barros LF, Da-Luz PL, Silveira MC, et al. Ventricular
fibrillation in acute experimental myocardial ischemia:
protection by magnesium sulfate. Braz J Med Biol Res
1988;21:791-9.
41. Crampton RS. Varying extracellular Mg2+ alters
ischemic and reperfusion tachyarrhythmias. (Abstract).
Circulation 1983;68:146.
42. Haverkarmp W, Hindricks G, Keteller T. Prophylactic
anti-arrhythmic and antifibrillatory effects of intravenous
magnesium sulphate during acute myocardial ischemia. (Abstract).
Eur Heart J 1988;9:228.
43. Chang C, Varghese PJI Downey J, et al. Magnesium
deficiency and myocardial infarct size in the dog. J Am Coll
Cardiol 1985;5:280-9.
44. Shine KI. Myocardial effects of magnesium. Am J Physiol
1979;237:H413-23.
45. Altura BM, Zhang A, Altura BT. Magnesium, hypertensive
vascular diseases, atherogenesis, subcellular compartmentation of
Ca2+ and Mg2+ and vascular contractility.
Miner Electrolyte Metab 1993;19:323-36.
46. Arsenian MA. Magnesium and cardiovascular disease. Prog
Cardiovasc Dis 1993;35:271-310.
47. Teo KK, Yusuf S. Role of magnesium in reducing mortality
in acute myocardial infarction: a review of the evidence. Drugs
1993;46:347-59.
48. Horner SM. Efficacy of intravenous magnesium in acute
myocardial infarction in reducing arrhythmias and mortality:
meta-analysis of magnesium in acute myocardial infarction.
Circulation 1992;86:774-9.
49. Casscells W. Magnesium and myocardial infarction. Lancet
1994;343:807-9.
50. Egger M, Smith GD. Misleading meta-analysis. (Editorial).
BMJ 1995;310:752-4.
51. ISIS-4 Collaborative Group. Fourth International Study of
Infarct Survival: protocol for a large simple study of the
effects of oral mononitrate, of oral captopril, and of
intravenous magnesium. Am J Cardiol 1991;68:87D-IOOD.
52. Seelig MS, Elin RJ. Reexamination of magnesium infusions
in myocardial infarction. (Editorial). Am J Cardiol 1995;76:
172-3.
53. Antman EM. Magnesium in acute MI: timing is critical.
(Editorial). Circulation 1995;92:2367-72.
54. Antman EM. Randomized trials of magnesium in acute
myocardial infarction: big numbers do not tell the whole story.
(Editorial). Am J Cardiol 1995;75:391-3.
55. Galloe AM, Rasmussen HS, Jorgensen LN, et al. Influence of
oral magnesium supplementation on cardiac events among survivors
of an acute myocardial infarction.,BMJ 1993;307: 585-7.
56. Singh RB. Effect of dietary magnesium supplementation in
the prevention of coronary heart disease and sudden cardiac
death. Magnes Trace Elem 1990;9:143-51.
57. Sharrett AR. The role of chemical constituents of drinking
water in cardiovascular diseases. Am J Epidemiol
1979;110:401-19
58. Eisenberg MJ. Magnesium deficiency and sudden death. Am
Heart J 1992;124:544 -9.
59. Chipperfield B, Chipperfield JR. Heart muscle magnesium,
potassium, and zinc concentrations after sudden death from heart
disease. Lancet 1973;2:293-6.
60. Anderson TW, Neri LC, Schreiber GB, et al. Ischemic heart
disease, water hardness and myocardial magnesium. (Letter). Can
Med Assoc J 1975;113:199-203.
61. Chipperfield B, Chipperfield JR, Behr G, et al. Magnesium
and potassium content of normal heart muscle in areas of hard and
soft water. Lancet 1976;1:121-2.
62. Chipperfield B, Chipperfield JR. Differences in metal
content of the heart muscle in death from ischemic heart disease.
Am Heart J 1978;95:732-7.
63. Johnson CJ, Peterson DR, Smith EK. Myocardial tissue
concentrations of magnesium and potassium in men dying suddenly
from ischemic heart disease. Am J Clin Nutr 1979;32:967-70.
64. Chipperfield B, Chipperfield JR. Relation of myocardial
metal concentrations to water hardness and death rates from
ischaemic heart disease. Lancet 1979;2:709-12.
65. Elwood PC, Sweetnam PM, Beasley WH, et al. Magnesium and
calcium in the myocardium: cause of death and area differences.
Lancet 1980;2:720-2.
66. Seelig MS. Magnesium deficiency in the pathogenesis of
disease: early roots of cardiovascular, skeletal, and renal
abnormalities. New York, NY: Plenum Medical Book Company,
1980.
67. Kobayashi JA. On the geographical relationship between the
chemical nature of river water and the death rate from apoplexy.
Berichte Ohara Inst Landwirtschaft Biol (Okayama)
1957;11:12-21.
68. Punsar S. Cardiovascular mortality and quality of drinking
water: an evaluation of the literature from an epidemiological
point of view. Work Environ Health 1973; 10: 107-25.
69. Neri LC, Hewitt D, Schreiber GB. Can epidemiology
elucidate the water story? Am J Epidemiol 1974;99:75-88
70. Comstock GW. Water hardness and cardiovascular diseases.
Am J Epidemiol 1979;1 10:375-400.
71. Biersteker K. Water hardness and mortality. (In Dutch).
Tidsskr Soc Geneesk 1967;45:658-61.
72. Bjørck G, Bostrøm H, Widstrøm A. On
the relationship between water hardness and death rates in
cardiovascular disease. Acta Med Scand 1965;178:239-52.
73. Blachly PH. Lithium content of drinking water and ischemic
heart disease. N Engl J Med 1969;281:682.
74. Crawford T, Crawford MD. Prevalence and pathological
changes of ischaemic heart disease in a hard-water and in a
soft-water area. Lancet 1967;1:229-32.
75. Dudley EF, Beldin RA, Johnson BC. Climate, water hardness
and coronary heart disease. J Chronic Dis 1969;22:25-48.
76. Elwood PC, Abernethy M, Morton M. Mortality in adults and
trace elements in water. Lancet 1974;2:1470-2.
77, Hart JT. The distribution of mortality from coronary heart
disease in South Wales. J R Coll Gen Pract 1970;19:258-68.
78. Lindemann RD, Assenzo JR. Correlation between water
hardness and cardiovascular death in Oklahoma counties. Am J
Public Health 1964;54:1071-7.
79, Morris J, Crawford MD, Heady JA. Hardness of local water
supplies and mortality from cardiovascular disease. Lancet 196
1;1: 860-2.
80. Nerbrand C, Svardsudd K, Ek J, et al. Cardiovascular
mortality and morbidity in seven counties in Sweden in relation
to water hardness and geological settings. Eur Heart J
1992;13:721-7
81. Roberts CJ, Lloyd S. Association between mortality from
ischaemic heart disease and rainfall in South Wales and in the
county boroughs of England and Wales. Lancet 1972;1:1091-3.
82. Sauer HI, Parke DW, Neill M. Associations between drinking
water and death rates. In: Hemphill DD, ed. Trace substances in
environmental health: Missouri's 4th annual conference on trace
substances in environmental health. Columbia, MO: University of
Missouri-Columbia, 1971:318-25.
83. Scassellati-Sforzolini G, Paseasio F. Epidemiological
correlation between water hardness and cardiovascular mortality.
(In Italian). Ann Sanita Pubblica 1971;32:45-60.
84. Schroeder HA. Municipal drinking water and cardiovascular
death rates. ]AMA 1966;195:81-5.
85. Schroeder HA, Kraemer LA. Cardiovascular mortality,
municipal water, and corrosion. Arch Environ Health 1974;28:
303-11.
86. Shaper AG, Packham RF, Pocock SJ. The British Regional
Heart Study: cardiovascular mortality and water quality. J
Environ Pathol Toxicol 1980;4:89-111.
87. Voors AW. Minerals in the municipal water and
atherosclerotic heart death. Am J Epidemiol 1971;93:259-66.
88. Winton EF, McCabe LJ. Studies relating to water
mineralization and health. J Am Water Works Assoc 1970;62:
26-30.
89. Schroeder HA. Relation between mortality from
cardiovascular disease and treated water supplies. JAMA 1960;172:
1902-8.
90. Neri LC, Mandel JS, Hewitt D. Relation between mortality
and water hardness in Canada. Lancet 1972;1:931-4.
91. Shaper AG, Elford J. Regional variations in coronary heart
disease in Great Britain: risk factors and changes in
environment. In: Marmot MG, Elliott P, eds. Coronary heart
disease epidemiology: from aetiology to public health. New York,
NY: Oxford University Press, 1992:127-39.
92. Hopps HC, Feder GL. Chemical qualities of water that
contribute to human health in a positive way. Sci Total Environ
1986;54:207-16.
93. Goldsmith NF, Goldsmith JR. Epidemiological aspects of
magnesium and calcium metabolism: implications of altered
magnesium metabolism in women taking drugs for the suppression of
ovulation. Arch Environ Health 1966;12:607-19.
94. Schroeder HA. Relations between hardness of water and
death rates from certain chronic and degenerative diseases in the
United States. J Chronic Dis 1960;12:586-91.
95. Karppanen H, Pennanen R, Passinen X. Minerals, coronary
heart disease, and sudden coronary death. Magnes Bull
1978;12:80-6.
96. Karppanen H. Ischemic heart disease: an epidemiological
perspective with special reference to electrolytes. Drugs
1984;28(suppl 1):17-27.
97. Marier JR, Neri LC. Quantifying the role of magnesium in
the interrelationship between human mortality/morbidity and water
hardness. Magnesium 1985;4:53-9.
98. Luoma H, Helminen SK, Ranta H, et al. Relationships
between the fluoride and magnesium concentrations in drinking
water and some components in serum related to cardiovascular
diseases in men from four rural districts in Finland. Scand J
Clin Lab Invest 1973;32:217-24.
99. Allwright SP, Coulson A, Detels R. Mortality and water
hardness in three matched communities in Los Angeles. Lancet
1974;2:860-4.
100. Leary WP, Reyes AJ, Lockett CJ, et al. Magnesium and
deaths ascribed to ischaemic heart disease in South Africa: a
preliminary report. S Afr Med J 1983;64:775-6.
101. Leary WP. Content of magnesium in drinking water and
deaths from ischaemic heart disease in white South Africans.
Magnesium 1986;5:150-3.
102. Teitge JE. Incidence of myocardial infarct and the
mineral content of drinking water. (In German). Z Gesamte Inn Med
1990;45:478-85.
103. Rylander R, Bonevik H, Rubenowitz E. Magnesium and
calcium in drinking water and cardiovascular mortality. Scand J
Work Environ Health 1991;17:91-4.
104. Luoma H, Aromaa A, Helminen S, et al. Risk of myocardial
infarction in Finnish men in relation to fluoride, magnesium and
calcium concentration in drinking water. Acta Med Scand
1983;213:171-6.
105. Durlach J, Bara M, Guiet-Bara A, et al. Relationship
between magnesium, cancer and carcinogenic or anticancer metals.
Anticancer Res 1986;6:1353-61.
106. Punsar S, Karvonen MJ. Drinking water quality and sudden
death: observations from West and East Finland. Cardiology
1979;64:24-34.
107. Altura BM, Altura BT. Role of magnesium in the
pathogenesis of hypertension, updated: relationship to its
actions on cardiac, vascular smooth muscle, and endothelial
cells. In: Laragh JH, Brenner BM, eds. Hypertension:
pathophysiology, diagnosis, and management. 2nd ed. New York, NY:
Raven Press, 1995:1213-42.
108. King J. The need to consider functional endpoints in
defining nutrient requirements. San Francisco, CA: Western Human
Nutrition Center, 1994:5.
109. Schwartz R, Spencer H, Wentworth RA. Measurement of
magnesium absorption in man using stable 26 Mg as a
tracer. Clin Chim Acta 1978;87:265-73.
110. Hardwick LL, Jones MR, Buddington RK, et al. Comparison
of calcium and magnesium absorption: in vivo and in vitro
studies. Am J Physiol 1990;259:G720-6.
111. Durlach J. Recommended dietary amounts of magnesium: Mg
RDA. Magnes Res 1989;2:195-203.
112. Lachance P, Langseth L. The RDA concept: time for a
change? Nutr Rev 1994;52:266-70.
113. Hedges LV, Olkin I. Estimation of effect size when not
all study outcomes are observed. In. Hedges LV, Olkin I, eds.
Statistical methods for meta-analysis. Boston, MA: Academic
Press, Inc, 1985:285-309.
114. Rosenthal L. The "file drawer problem" and tolerance for
null results. Psychol Bull 1979;86:638-61.
115. Morgenstern R. Uses of ecologic analysis in epidemiologic
research. Am J Public Health 1982;72:1336-44.
116. Blalock HM, Jr. Causal inferences in nonexperimental
research. Chapel Hill, NC: University of North Carolina Press,
1964.
117. Karppanen H. An antihypertensive salt: crucial role of
Mildred Seelig in its development. J Am Coll Nutr
1994;13:493-5.
Received for publication June 15, 1995, and in final form
March 11,1997.
Abbreviations: ICD-9-CM, International Classification of
Diseases, Ninth Revision, Clinical Modification; IHD, ischemic
heart disease; ISIS-4, Fourth International Study of Infarct
Survival; RDA, Recommended Dietary Allowance.
From the Division of Environmental and Occupational Disease
Control, California Department of Health Services, Emeryville,
CA.
Arthur Marx and Raymond R. Neutra
This page was first uploaded to The Magnesium Web Site on
January 26, 2002
http://www.mgwater.com/


