Magnesium 6: 18-27 (1987)
Magnesium Intake during Pregnancy
Kay B. Franz
Department of Food Science and Nutrition, Brigham Young
University, Provo, Utah, USA
This paper was presented at the 4th International Symposium on
Magnesium, July 23-28, 1985, Blacksburg, Va, USA.
Key Words. Pregnancy · Diet ·
Nutrient density · Drinking water · Protein
· Magnesium · Preeclampsia
Abstract. The mean dietary magnesium intake
of pregnant women is 35-58% of the recommended dietary
allowance of 450 mg. Low-income women consumed 97-100 mg
magnesium/1,000 kcal while women with higher incomes averaged
120 mg/1,000 kcal. Diets high in fat and sugar and low in whole
grains, vegetables and fruits have a lower magnesium density.
Magnesium content of water can also make a significant
contribution to magnesium intake. Magnesium from prenatal
supplements, if present, is seldom more than 100 mg. Additional
supplementation is needed for adequate magnesium nutriture
during pregnancy.
Introduction
There is a growing literature which provides evidence that a
compromised magnesium nutritional status may be involved in
several disorders that can occur during pregnancy. These include
hypertension [1, 10, 11, 19], vasospasm [1, 2, 27], coagulation
defects [49], premature delivery [12, 26], intrauterine growth
retardation [10-12, 26] and muscle cramping [4, 9, 16, 31].
Magnesium nutritional status can be influenced by a number of
factors, but the easiest to correct is magnesium intake. In
addition, dietary factors can modulate the absorption of the
amount of magnesium ingested.
Before discussing magnesium intake during pregnancy, and
factors that affect it, I would like to draw attention to
previous reviews of this subject by Seelig [38, 42]. Her studies
have provided an indepth review of material published prior to
1980. Rather than summarizing her work, I will address work
published since then and, hopefully, provide some additional
insights into the subject.
Magnesium Intake
Diet
There have been six studies, published since 1980, that have
reported magnesium intake during pregnancy in the United States
(table I). The mean magnesium intakes from these studies have
ranged from 158 to 259 mg a day when no supplements were taken.
These intakes are 35-58% of the recommended dietary allowance
(RDA) of 450 mg [18].
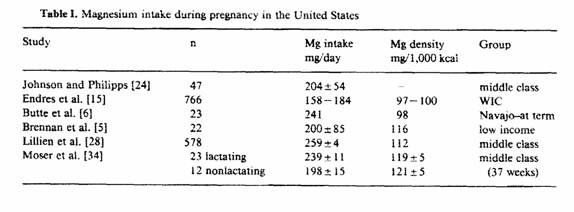
Johnson and Philipps [24] estimated the magnesium intake in 47
pregnant women during the fall and winter of 1974. Each women
recorded her food intake every 8th day from the 5th month until
delivery. This provided a total of 568 dietary records. Magnesium
intakes ranged from 103 to 333 mg/day with an average of 204
± 54 mg (± SD). None reached the recommended
allowance of 450 mg. Two cases of toxemia occurred. The daily
magnesium intakes of these two women were estimated to be 178 and
186mg.
In 1981, Endres et al, [15] reported the magnesium intake of
pregnant women participating in a supplemental food program in
1978-79. Nutrient intakes were estimated from 24-hour recalls
obtained by trained interviewers from 10% of participants in the
Special Supplemental Food Program for Women, Infants, and
Children (WIC) in the state of Illinois. This program is
sponsored by the US government. Of the 766 women, 15% had been
receiving supplemental foods for at least 6 months, 85% were
applicants to the program. Estimated magnesium intakes of WIC
applicants 35 ± 19% of the RDA of 450 mg. Those
participating in the WIC program averaged 41 ± 21% of the
RDA. This translates to 158 and 184 mg magnesium per day.
Butte et al. [6] in 1981 reported the magnesium intake of
pregnant Navajo women. These women were from a rural, semiarid
part of Arizona in the southwestern part of the United States and
were planning to breastfeed their infants. Typical food intakes
were determined using a questionnaire and by a 24-hour dietary
recall at term. The median magnesium intake was 230 mg, which is
only 51% of the RDA. The mean daily magnesium intake was 241
± 114 mg. Diets reflected few fruits and vegetables and
frequent soft drinks. Prenatal supplements were prescribed which
would provide an additional 100mg of magnesium, if used.
Brennan et al. [5] analyzed duplicate food composites
collected from 22 pregnant low-income women who attended an
obstetrical clinic in an urban area. Food composites were
collected with a nutritionist present in the participant’s
home. Even though women were instructed ‘to prepare and
consume meals in the usual manner’, the presence of the
nutritionist could have influenced food choices. Mean magnesium
intake was 200 ± 85 mg.
Magnesium intake was determined by Lillien et al. [28] as part
of a study evaluating diet and ethanol intake during pregnancy.
Diet records were determined by a 24-hour recall type interview
1-4 days postpartum. Subjects were asked to recall food intake of
an ‘average day’. The mean magnesium intake from diet
alone for the 578 women was 259 ± 4 mg. When supplements
were included, magnesium intake was 316 ± 5 mg.
In the study of Moser et al. [34], 3-day dietary records were
kept by 35 middle-class women during the 37th week of pregnancy.
Those women who were planning to nurse their infants had a mean
magnesium intake of 239 ± 11, while those not planning to
nurse had a mean intake of 198 ± 15 mg. When compared on
the basis of nutrient density, the magnesium content of the diets
were the same. This shows that those women who were not going to
nurse their infants were eating less total food, which was
reflected in a decreased magnesium intake.
Since none of the mean intakes of the previous studies reached
the RDA, it is possible that the RDA is too high. This
possibility was indirectly tested in an extensive magnesium
balance study with 10 pregnant women [3]. The goal was to perform
two 7-day balance studies on all 10 women during each trimester
of pregnancy, but this was not achieved in all cases. In all, 47
7-day balance periods were obtained. Magnesium intake averaged
269 ± 55 mg/day. Negligible amounts were provided by
prenatal supplements. Only 3 of the 47 balances were positive for
magnesium. Magnesium balance averaged -40 ± 50 mg/day. If
it were assumed that these negative balances occurred for 200 of
the 280 days of pregnancy, the women would have lost 8 g of
magnesium during pregnancy. Since it has been estimated that a
70-kg individual contains 20-25 g of magnesium in the entire
body, an 8-gram loss is significant.
The results of this balance study suggest that average
magnesium intakes are not adequate for pregnant women. No
justification for lowering the RDA below 450 mg can be made.
Nutrient Density
When the diets previously described were analyzed on the basis
of magnesium nutrient density (table I), low-income women had
magnesium intakes of 97-100 mg/1,000 kcal. As the income
increased, magnesium nutrient density increased. Those women from
middle-class families had magnesium intakes of 119-121 mg/1,000
kcal. This suggests that women with more money are able to buy
food that has greater magnesium nutrient density.
The magnesium density of common foods consumed in the United
States is illustrated in table II. Note that foods with nutrient
densities of less than 50 mg/1,000 kcal are high in fat or sugar.
As the nutrient density increases, foods contain less fat and
sugar. Whole grains and legumes appear. Vegetables and fruits
gradually dominate the foods with the highest nutrient density,
because these foods are inherently low in calories.
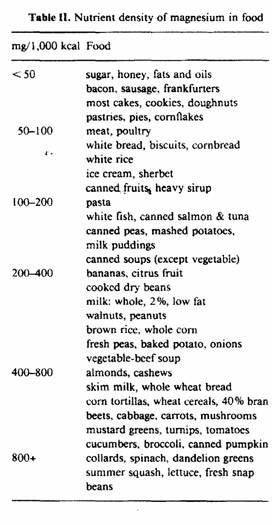
Dietary magnesium intake reflects the nutrient density of
individual food choices. Diets low in magnesium density would
contain refined cereal grains, fatty meats, high amounts of fats
and sugars, few fruits and vegetables and sugar-containing soft
drinks. Diets high in magnesium density would contain whole
grains, lean meats, low amounts of fats and sugars, abundant
fruits and vegetables, and low-fat milk. It is possible to have
diets which contain more than 200 mg/1,000 kcal if wise choices
are made.
Chadhuri [7] observed that toxemic women preferred more
refined cereal grains, fewer leafy green vegetables and more fat
than women who did not develop toxemia. Chung et al. [8] found
that toxemic women chose diets higher in fat and cholesterol than
women without toxemia.
Water Hardness
It has been estimated that magnesium intake from 2 liters of
water can range from 2 to more than 50 mg/day depending upon the
hardness of the water [35]. Johnson and Philipps [24] estimated
that the pregnant women in their study in Wisconsin ingested
about 35 mg of magnesium a day from the water.
Water hardness in the United States varies from very soft to
very hard [14]. Water hardness is expressed in terms of calcium
content but magnesium can make a substantial contribution to its
hardness. Those states with soft water are found in the
northwest, New England, and in the southeastern parts of the
country.
Maternal deaths attributed to toxemia of pregnancy from 1961
to 1965 in the United States were 6.2/100,000 live births [17].
This is the national average, but there was a wide range
deviating from it, depending upon where the woman lived. Some
states had half the national average while others exceeded the
national average. Toxemic deaths in Mississippi were nearly five
times the national average. The highest incidence of toxemic
deaths occurred in the southeastern part of the United States.
These are the states with the softest water.
It has been noted that per capita income of states is
inversely related to deaths from toxemia [17]. If states were
divided into three groups based on their per capita income from
1961 to 1965, those which would be in the upper third of the
income groups had a toxemic death rate of 61% of the national
average. Those states in the middle third of the per capita
income groups had a toxemic death rate of 95% of the national
average, while those states in the lowest third of the income
groups had a toxemic death rate of 192% of the national average.
The poorest state is Mississippi which had a toxemic death rate
of 487% of the national average.
There are eleven states with soft water where deaths from
toxemia were less than 85% of the national average. Nine of these
states had a high per capita income. These states are
Connecticut, Delaware, New York, Massachusetts, Maryland,
Washington, Rhode Island, Oregon, and New Jersey. Only Vermont
and Maine did not have high per capita incomes. Conversely, there
were nine states with toxemic deaths of more than 150% of the
national average. Seven of these states are in soft-water areas.
Of these seven, six rank in the lowest third of the per capita
income groups and one is in the middle third. The six states,
Georgia, North Carolina, Alabama, Arkansas, South Carolina and
Mississippi, are in the southeastern part of the country. The
seventh state, New Hampshire, is in New England. The remaining
two states with high rates of toxemic deaths are Tennessee and
Texas. Tennessee has moderately soft water and is in the lowest
third of the per capita income groups. Texas has hard water and
is in the middle third of the income groups, but it has a large
low-income population.
The average temperature of the state may also be associated
with toxemic deaths. The lowest incidence of toxemic deaths
occurs in the northern states. The further south the states are
located, the more the toxemic death rate increases. The highest
toxemic death rates generally occur in the most southern states
at every latitude. Since the average temperature rises as the
location of the state is further south, sweat losses of magnesium
may be an important factor. The highest average temperatures
would be in the southern states: the same states where the
toxemic death rate was the highest. This may be a factor in Texas
which contributes to the increased maternal death rate related to
toxemia even though the state has hard water.
By putting water hardness, per capita in come, mean
temperature and toxemic deaths together, the environment where
toxemia occurs becomes evident. Toxemic deaths would have the
highest incidence where (1) per capita income is low, (2) the
mean temperature is elevated, and (3) magnesium content of the
water is low.
A low per capita income would limit the ability of the woman
to buy foods with a high magnesium density. I have previously
shown that low-income women have a lower nutrient density of
magnesium in their diet. With an elevated mean temperature, there
would be increased sweat losses. A low-income mother would not be
able to afford housing with air conditioning, which would help to
minimize sweat losses. Sweat losses of magnesium may be
considerable depending upon the mean temperature [13, 38].
Magnesium content of drinking water would only be important if
the mother is in a marginal magnesium state. This may occur with
a low per capita income and a high mean environmental
temperature. Now, the magnesium content of the water could be
crucial. If the water were soft with essentially no magnesium,
the mother would develop toxemia. If the water were hard with a
high level of magnesium, it could be protective against
development of toxemia.
In Great Britain, it has been observed that there is less
preeclampsia and eclampsia in London than in other parts of the
country [23]. London also has hard water [32].
Supplements
Not all prenatal supplements used in the United States contain
magnesium. Within the last few years, more include magnesium than
previously. Magnesium is usually included in the form of
magnesium oxide. Magnesium oxide is 60% magnesium by weight.
If magnesium is included in a prenatal supplement, it is
normally added in amounts of 100mg of elemental magnesium. There
is no information about the bioavailability of magnesium
contained in these supplements. Prenatal supplements do not
provide enough magnesium to meet the RDA since most dietary
intakes are less than 300 mg. Additional magnesium
supplementation for pregnant women is recommended.
Factors Which Modulate Absorption of Magnesium
Several factors have been implicated in modulating magnesium
absorption from the intestine. These have been reviewed
extensively by Seelig [38-41], Lindeman [29], and Tansy and
Kendall [46].
Protein
It has been quoted for a long time that increasing protein in
the diet will increase magnesium absorption. This is based on the
work of McCance et al. [33] in 1942. They fed diets which
contained 0.7-0.9 g of protein/kg body weight and found a
magnesium absorption of 32%. When the diets contained 2.3-2.6 g
of protein/kg body weight, magnesium absorption was 41%. These
diets also contained fiber and phytic acid from vegetables and
92% extraction wheatmeal bread.
Hunt and Schofield [21] fed women diets in which the protein
intake varied from 20 to 48 g. Magnesium absorption varied from
28 to 53% with the highest absorption occurring in diets with 48
g of protein. When this study is analyzed on the basis of grams
of protein per kilogram of body weight, protein intake with a
mean of 0.36 g/kg body weight resulted in a 28% absorption of
magnesium. Mean protein intakes of 0.46, 0.59 and 0.76 g/kg body
weight resulted in magnesium absorptions of 46, 42 and 53%,
respectively. Essentially, protein intakes of more than 0.4 g/kg
body weight resulted in magnesium absorptions of more than 40%.
These diets were low in phytic acid but fiber was present from
fruits and vegetables.
Mahalko et al. [30] arrived at similar findings. They fed men
diets that contained 0.86 and 1.24 g of protein per kilogram body
weight and obtained magnesium absorptions of 51.5 and 52.7%,
respectively. These diets were also low in phytic acid. With such
levels of protein intake, an increase in magnesium absorption
would not be expected, especially if magnesium absorption was
already maximal.
Fat
Fat in the diet may act in two different ways. A high-fat
intake can dilute the magnesium nutrient density of the diet and
has been shown to reduce the absorption of magnesium [29, 38-4l].
More recent studies showed that, while fat reduced magnesium
absorption in individuals with malabsorption syndromes [20]
varying the fat intake from 22 to 42% of the energy intake in
normal men had no influence on magnesium absorption [48]. The
reason for the apparent conflict in the literature is not
evident.
Fiber and Phytic Acid
Fiber can reduce magnesium absorption [22, 25, 36, 47]. Phytic
acid has also been implicated in reducing magnesium absorption
[36, 37, 41].
Diets high in fiber and phytic acid may result in decreased
absorption of magnesium. Fortunately, foods which contain fiber
and phytic acid usually also contain higher amounts of
magnesium.
Calcium and Phosphorus
A paradox occurs in discussing the influence of calcium and
phosphorus on magnesium absorption. Calcium and phosphorus have
been reported to decrease magnesium absorption [29, 38-41, 46],
but Spencer et al. have shown no influence of calcium [43, 45] or
phosphorus [44] on magnesium absorption in men with magnesium
intakes typical of American diets.
Among a group of pregnant women (24 years of age and older) we
were studying, we found that those women with the most muscle
cramping during pregnancy had lower magnesium intakes and higher
calcium and phosphorus intakes than women with less cramping.
Ratios of Ca/Mg and P/Mg intakes were 4.51 ± 0.79 and 4.71
± 0.63, respectively, in women with most muscle cramping
and 3.70 ± 0.79 and 4.06 ± 0.54, respectively, in
the women with less cramping. These Ca/Mg and P/Mg ratios were
significantly different between the two groups of women (p =
0.025; p = 0.011; n = 25).
These data make me question the common recommendation of a
quart of milk daily for pregnant women. A quart of skim milk
would provide 1,200 mg of calcium, 980 mg of phosphorus, 120 mg
of magnesium, and has Ca/Mg and P/Mg ratios of 10.0 and 8.7,
respectively. These are in excess of similar ratios (2.7) derived
from the RDA and based on magnesium at 450 mg and calcium and
phosphorus at 1,200 mg each [18]. In addition, prenatal
supplements commonly provide 200 mg of calcium. With a quart of
milk, a prenatal supplement and other foods in the diet, calcium
and phosphorus intakes would be excessive for the magnesium
content. A recommendation of 2 - 2 1/2 cups of milk when combined
with the prenatal supplement and other foods in a balanced diet
will meet the RDA for calcium and phosphorus for pregnancy and
provide better Ca/Mg and P/Mg ratios.
Since calcium and phosphorus are highly correlated in diets (p
<0.001), it is not completely clear whether it is calcium or
phosphorus that influences magnesium absorption most. Since the
P/Mg ratio of the food intakes of the pregnant women in our study
was more significant that the Ca/Mg ratio, phosphorus may be more
important than calcium. Further study is needed in this area.
Conclusions
Magnesium intake of pregnant women needs to be increased by
either dietary means or supplements, especially among low-income
women in soft-water states. Excessive intakes of calcium and
phosphorus may be detrimental to magnesium absorption.
L’absorption de magnésium an cours de la
grossesse
L’absorption moyenne de magnesium avec le régime
chez les femmes enceintes est de 35-58% de la ration alimentaire
recommandée de 450 mg. Les femmes avec un faible revenu
ant consommé 97-100 mg/1000 kcal, alors que les femmes
avec des revenus plus élevés ont consommé en
moyenne 120 mg/ 1000 kcal. Les régimes riches en graisses
et en sucres et pauvres en céréales completes, en
végetaux et en fruits présentent une densité
plus faible du Mg. La teneur en Mg de l’eau peut aussi
contribuer significativement à la consommation de Mg. Le
Mg des produits supplémentaires prénataux,
s’il est present, est rarement supérieur à
100 mg. Un apport supplémentaire est nécessaire
pour une nutrition adequate en magnesium au cours de la
grossesse.
References
1 Altura, B.M.; Altura, B.T.; Carella, A.: Magnesium
deficiency-induced spasms of umbilical vessels: relation to
preeclampsia, hypertension, growth retardation. Science
221: 376-378 (1983).
2 Altura, B.M.; Altura, B.T.; Gebrewold, A.; Ising, H.;
Gunther, TI: Magnesium deficiency and hyper tension: correlation
between magnesium-deficient diets and microcirculatory changes in
situ. Science 223: 1315-1317(1984).
3 Ashe, J.R.; Schofield, F.A.; Gram, M.R.: The retention of
calcium, iron, phosphorus, and magnesium during pregnancy: the
adequacy of prenatal diets with and without supplementation. Am.
J. clin. Nutr. 32: 286-291(1979).
4 Bartl, W.; Riss, P.: Zur Pathophysiologie und Therapie des
Magnesiummangels in der Schwangerschaft. Magnesium-Bull.
6: 60-62 (1984).
5 Brennan, R.E.; Kohrs, M.B.; Nordstrom, J.W.; Sauvage, J.P.;
Shank, RE.: Nutrient intake of low- income pregnant women:
laboratory analysis of food consumed. J. Am. diet. Ass.
83: 546-550 (1983).
6 Butte, N.F.; Calloway, D.H.; Van Duzen, J.L.: Nutritional
assessment of pregnant and lactating Navajo women. Am: J. clin.
Nutr. 34: 22 16-2228 (1981).
7 Chadhuri, S.K.: Dietetic deficiency in toxemia of pregnancy.
Indian Practner 22: 13 1-134 (1969).
8 Chung, R.; Davis, H.; Ma, Y.; Naivikul, O.; Williams, C.:
Wilson, K.: Diet-related toxemia in pregnancy. 1. Fat, fatty
acids, and cholesterol. Am. J. clin. Nutr. 32: 1902-1911
(1979).
9 Classen H.-G.; Helbig, J.: Magnesium in Geburtshilfe und
Gynäkologie. Magnesium-Bull. 6: 45-51 (1984).
10 Conradt, A.; Weidinger, H.; Algayer, H.: Evidence that
magnesium deficiency could be a causal factor of (essential)
gestosis; in Schenker, Rippmann, Weinstein, Recent advances in
pathophysiological conditions in pregnancy (Elsevier, New York
1984).
11 Conradt, A.; Weidinger, H.; Algayer. H.: On the role of
magnesium in fetal hypotrophy, pregnancy induced hypertension,
and pre-eclampsia. Magnesium-Bull. 6: 68-76 (1984).
12 Conradt, A.; Weidinger, H.; Algayer, H.: Magnesium therapy
decreased the rate of intrauterine fetal retardation, premature
rupture of membranes and premature delivery in risk pregnancies
treated with betamimetics. Magnesium 4: 20-28
(1985).
13 Costill, D.L.; Coté, R.; Fink, W.: Muscle water and
electrolytes following varied levels of dehydration in man. J.
appl. Physiol. 40: 6-11(1976).
14 Durfor, C.N.; Becker, E.: Chemical quality of public water
supplies of the United States and Puerto Rico, 1962; Hydrologic
investigations atlas HA-200 (US Geological Survey, Washington DC
1964).
15 Endres, J.M.; Sawicki, M.; Casper, J.A.: Dietary assessment
of pregnant women in a supplemental food program. J. Am. diet.
Ass. 79: 121-126 (1981).
16 Fehlinger, R.; Kemnitz, C.; Dreissig, P.; Egert, M.;
Seidel, K.: Frühgeburtlichkeit. tetanische
Reaktionsbereitschaft und Magnesiummangel: eine retrospektive
Untersuchung an 132 Müttern. Magnesium-Bull. 6:
52-59 (1984).
17 Food and Nutrition Board: Maternal nutrition and the course
of pregnancy (National Academy Sciences National Research
Council, Washington DC 1970).
18 Food and Nutrition Board: Recommended dietary allowances;
9th ed. (National Academy Sciences National Research Council,
Washington DC 1980),
19 Franz, K.B.; Mangum, K.C.; Hill, S.F.; Minton, S.D.: Effect
of magnesium supplementation during pregnancy on mean arterial
pressure at delivery (Abstract). J. Am. Clin. Nutr. 4:
376 (1985).
20 Hessov, I.; Andersson, H.; Isaksson, B.: Effects of a
low-fat diet on mineral absorption in small- bowel disease.
Scand. J. Gastroent. 18: 55 1-554 (1983).
21 Hunt, S.M.; Schofield, F.A.: Magnesium balance and protein
intake level in adult human female. Am. J. clin. Nutr.
22: 367-373 (1969).
22 Ismail-Beigi, F.; Reinhold, J.G.; Faraji, B.; Abadi, P.:
Effects of cellulose added to diets of low and high fiber content
upon the metabolism of calcium, magnesium, zinc and phosphorus by
man. J. Nutr. 107: 510-518 (1977).
23 Jeffcoate, T.N.A.: Pre-eclampsia and eclampsia: the disease
of theories. Proc. R. Soc. Med. 59:397-404 (1966). -
24 Johnson, N.E.; Philipps, C.: Magnesium content of diets of
pregnant women; in Cantin, Seelig, Magnesium in health and
disease, pp. 827-831 (Spectrum Press, New York 1980).
25 Kelsay, J.L.; Behall, K.M.; Prather, ES.: Effect of fiber
from fruits and vegetables on metabolic responses of human
subjects. 11. Calcium, magnesium, iron, and silicon balances. Am.
J. clin. Nutr. 32: 1876-1880 (1979).
26 Kuti, V.; Balazs, M.; Morvay, F.; Varenka, Z.;
Székely, A.; Szücs, M.: Effect of maternal magnesium
supply on spontaneous abortion and premature birth and on
intrauterine foetal development: experimental epidemiological
study. Magnesium- Bull. 3: 73-79 (1981).
27 Lee, M.1.; Todd, H.M.; Bowe, A.: The effects of magnesium
sulfate infusion on blood pressure and vascular responsiveness
during pregnancy. Am. J. Obstet. Gynec. 149: 705-708
(1984).
28 Lillien, L.J.; Huber, A.M.; Rajala, M.M.: Diet and ethanol
intake during pregnancy. 3. Am. diet. Ass. 81: 252-257
(1982).
29 Lindeman, R.D.: Nutritional influences on magnesium
homeostasis with emphasis on renal factors; in Cantin, Seelig,
Magnesium in health and disease, pp. 38 1-399 (Spectrum Press.
New York 1980).
30 Mahalko, J.R.; Sand stead, H.H.; Johnson, L.K.; Milne,
D.B.: Effect of a moderate increase in dietary protein on the
retention and excretion of Ca, Cu, Fe, Mg, P, and Zn by adult
males. Am. J. clin. Nutr. 37: 8-14 (1983).
31 Mangum, K.C.; Hill, S.F.; Wade, B.B.; Richards, D.O.;
Minton, S.D.; Franz, K.B.: Effect of age, parity and magnesium
supplementation on muscle cramping during pregnancy (Abstract).
J. Am. Coil. Nutr. 4: 375-376 (1985).
32 Masironi, R.; Pisa, Z.; Clayton, D.: Myocardial infarction
and water hardness in European towns. J. envir. Path. Toxicol.
4/2,3: 77-87 (1980).
33 McCance, R.A.; Widdowson, E.M.; Lehmann, H.: The effect of
protein intake on the absorption of calcium and magnesium.
Biochem. J. 36: 686-691 (1942).
34 Moser, P.B.; Issa, C.F.; Reynolds, R.D.: Dietary magnesium
intake and concentration of magnesium in plasma and erythrocytes
of postpartum women. J. Am. Coil. Nutr. 2: 387-396
(1983).
35 Neri, L.C.; Johansen, H.L.: Water hardness and
cardiovascular mortality. Ann. N.Y. Acad. Sci. 304:
203-219 (l9
36 Reinhold, J.G.; Hedayati, H.; Lahimgarzadeh, A.; Nasr. K.:
Zinc, calcium, phosphorus, and nitrogen balances of Iranian
villagers following a change from phytate-rich to phytate-poor
diets. Eco1ogy Food Nutr. 2: 157-162 (1973).
37 Reinhold, 3.0.; Faradji, B.; Abadi, P.; Ismail-Beigi, F.:
Decreased absorption of calcium, magnesium, zinc and phosphorus
by humans due to increased fiber and phosphorus consumption as
wheat bread. J. Nutr. 106: 493-503(1976).
38 Seelig, M.S.: The requirement of magnesium by the normal
adult. Am. J. clin Nutr. 14: 342-390 (1964).
39 Seelig, M Human requirements of magnesium; factors that
increase needs; in Durlach, I Symposium international sur les
deficits magnésiques en pathologie humaine. Rapports, vol.
1, pp. 11-38 (SGEMV, Vittel 1971).
40 Seelig, MS.: Magnesium deficiency in the pathogenesis of
disease (Plenum, New York 1980).
41 Seelig, M.S.: Magnesium requirements in human nutrition.
Magnesium-Bull. 3: suppl. 1, pp. 26-47 (1981).
42 Seelig, M.S.: Contribution of magnesium deficiency to
gestational and infantile disorders; in Zichello, Collona di
ginecologia e ostetricia, pp. 132-145 (Pozzi, Rome 1983).
43 Spencer, H.; Lesniak, M.; Gatza, C.A.; Kramer, L.; Norris,
C.; Coffey, J.: Magnesium-calcium interrelationships in man; in
Hemphill, Trace substances in environmental health, vol. 12, pp.
241-247 (1978).
44 Spencer, H.; Kramer, L.; Gatza, C.; Norris, C.; Coffey, J.:
Magnesium-phosphorus interrelations in man; in Hemphill, Trace
substances in environ mental health, vol. 13, pp. 401-407
(1979).
45 Spencer, H.; Lesniak, M; Gatza, C.A.; Osis, D.; Lender, M.:
Magnesium absorption and metabolism in patients with chronic
renal failure and in patients with normal renal function.
Gastroenterology 79: 26-34 (1980).
46 Tansy, M.F.; Kendall, F.M.: Magnesium and the
gastrointestinal tract. Magnesium-Bull. 3: suppl. 1a,
pp. 55-66 (1981).
47 Dokkum, W. van; Wesstra, A.; Schippers, F.A.: Physiological
effects of fibre-rich types of bread. 1. The effect of dietary
fibre from bread on the mineral balance of young men. Br. J.
Nutr. 47: 451- 460 (1982).
48 Dokkum, W. van; Cloughlery, F.A.; Hulshof, K.F.A.M.;
Oosterveen, L.A.M.: Effect of variations in fat and linoleic acid
intake on the calcium, magnesium and ii balance of young men.
Ann. Nutr. Metab. 27: 361-369 (1983).
49 Weaver, K.: A possible anticoagulant effect of magnesium in
preeclampsia; in Cantin, Seelig, Magnesium in health and disease,
pp. 833-838 (Spectrum Press, New York 1980).
Received: September 28, 1985
Accepted: January 20, 1986
Kay B. Franz, PhD,
2218 Smith Family Living Center,
Department of Food Science and Nutrition,
Brigham Young University,
Provo, UT 84602 (USA)
This page was first uploaded to The Magnesium Web Site on
August 16, 2002
http://www.mgwater.com/


