in Childhood Nutrition, F Lifshitz, ed. Boca Raton, FL 1995,
Chapt. 17:197-224
PRENATAL AND GENETIC MAGNESIUM DEFICIENCY IN CARDIOMYOPATHY;
POSSIBLE VITAMIN AND TRACE MINERAL INTERACTIONS
MILDRED S. SEELIG, M.D., M.P.H.
Adjunct Professor, Department of Nutrition, School of Public
Health, North Carolina University Medical Center, Chapel Hill,
N.C.
Adjunct Professor, Department of Community and Preventive
Medicine, New York Medical College, Valhalla, New York
The section headers of this paper are as follows:
Infantile cardiovascular lesions that have caused early
serious disease or death, and that can contribute to the common
arterial and cardiac diseases of later life have been studied for
roles of nutritional or genetic factors. It is proposed, here,
that absolute or relative dietary magnesium deficiency, which is
common, (1-4), might contribute to the complications of these
diseases, particularly in mothers and infants with genetic
variations in handling magnesium. Reviewed elsewhere is
substantial experimental evidence that diets low in magnesium,
but otherwise adequate, produce microangiopathy and resultant
cardiomyopathy (CMP), and that magnesium deficiency intensifies
the thrombogenicity and macroangiopathy caused by atherogenic
diets.(4-7) Analysis of reports on infants with cardiovascular
lesions resembling those of ischemic heart disease of later life
has disclosed abnormalities resembling those of induced magnesium
deficiency.(4-8) Data on maternal magnesium inadequacy, as
contributory to poor outcomes of pregnancy, have been considered
elsewhere.(8-12) Familial patterns of the common cardiovascular
diseases suggest that attention be paid to genetic factors in
magnesium utilization in afflicted families. Severe metabolic
errors, such as isolated malabsorption,(13-17) and renal tubular
wasting of magnesium,(18-20) that are associated with
hypomagnesemia usually requiring parenteral magnesium therapy to
control convulsive and tetanic manifestations, have been shown to
be familial.(15,17-20) That such abnormalities may not be an "all
or none" phenomenon is suggested by genetic differences in plasma
and cellular magnesium levels that have been elicited in
different ethnic groups, in Types A and B subjects, in twin
studies, and in genetically selected mice.(21-23) Not studied is
whether these differences might be contributed to by
malabsorption, renal wasting, or differences in membrane
permeability to magnesium.
Since magnesium acts like a trace mineral as a cofactor for
over 300 enzymes,(24,25) its possible contributory role in
genetic diseases involving trace minerals and vitamins, in which
arterial disease develops, should be explored. Suggested here is
the possibility that the microangiopathy of the common genetic
disease, diabetes mellitus, and the cardiomyopathies seen in
several inborn errors of metabolism that entail
vitamin-dependencies and abnormal mineral metabolism, might also
involve function of enzymes in which magnesium is a cofactor.
Magnesium deficiency that results from generalized intestinal
malabsorption - whether caused by chronic diarrhea, Crohn's
disease, steatorrhea, celiac disease, or sprue, has long been
recognized,(26) but is not the subject of this report. The
metabolic error: isolated defect of intestinal absorption of
magnesium, was first identified in a French Canadian
baby.(13,27,28) Soon thereafter it was recognized in
American,(29) French,(14-20) Swedish,(16,17) and Italian(30)
infants, and then found to be familial when siblings developed
the same syndrome.(15,17) As a result of efforts to repair the
hypocalcemia by calcium, high dose vitamin D, and parathyroid
hormone therapy, in the first identified case of magnesium
malabsorption, glomerular and renal interstitial fibrosis
developed, with calcification, which was described as a major
complication of infantile hypomagnesemia.(13,27,28)
The risk of producing hypercalcemia by calcemic treatment of
hypocalcemia in the presence of hypomagnesemia, was cautioned
against in an early study of magnesium malabsorption.(26) That
low serum magnesium levels could not be relied upon for diagnosis
of severe magnesium deficiency of intestinal malabsorption was
commented on in a 1981 study of 17 hospitalized patients with
severe Crohn's disease, only six of whom had overt
hypomagnesemia, but 15 of whom had low urine magnesium.(31)
More frequently diagnosed (generally in older children and in
adults) is renal wastage of magnesium,(18-20,32-48) usually but
not always in association with hypokalemia or hypocalcemia or
both. Two families with members suffering from hypomagnesemia
caused by renal magnesium loss were reported in 1966.(18,19) Each
renal magnesium wasting syndrome has been reported in more than
one family member.(18-20,33,41,43,45,47)
Hypochloremic Hypokalemic Alkalosis with Magnesium
Wasting - Bartter's syndrome was diagnosed in a three
month old febrile, sweating baby with apathy, weakness and growth
failure, and laboratory findings characteristic of that syndrome:
hypokalemic hypochloremic alkalosis, hyper-reninism,
aldosteronism, and urinary wasting of sodium, as well as of
potassium and chloride.(34) Despite only marginally low serum
magnesium: mean of 1.7 mEq/L (range: 1.5-1.9), oral magnesium
supplementation (6 mg of Mg/kg/day) was prescribed because a
muscle biopsy disclosed subnormal magnesium levels (11.6 mEq/kg
fat-free weight; normal = 20 +/-1.8). A renal biopsy disclosed
hyperplasia of the juxtaglomerular apparatus and cloudy swelling
of the proximal tubules. Sustained magnesium supplements lowered
his high renin and aldosterone levels and corrected all of the
electrolyte and clinical abnormalities, except for slow growth,
by 16 months of age. Since this baby's magnesium deficit was not
detectable by serum values, the authors suggested that low
intracellular magnesium might be more common than suspected in
Bartter's syndrome. In another family(47) three siblings had
Bartter's syndrome with pronounced hypomagnesemia; necropsy
findings of juxtaglomerular hyperplasia were similar to that
reported from a renal biopsy from the infant with low muscle
magnesium.(34) Renal magnesium wasting has been reported by
others in patients with hypokalemic alkalosis as part of
Bartter's syndrome [20,40]. In three siblings (two girls and a
boy) with familial hypokalemic alkalosis with tubulopathy, rather
than the glomerular abnormality generally considered part of
Bartter's syndrome, oral magnesium supplements of 40-60 mEq/day
as MgCl2 corrected the potassium loss, but had no effect on the
elevated renin, and raised the aldosterone levell(41) The
hypomagnesemia of two adult sisters in another family was
associated with hypokalemic alkalosis, normotensive
hyper-reninemism, and marginally high mineralocorticoids.(18)
Renal Tubular Acidosis with Magnesium Wasting
- Renal magnesium leakage has also been associated with renal
tubular acidosis, usually with hypocalcemia, hypercalciuria and
nephrocalcinosis(33,39,40,45) and/or
chondro-calcinosis.(32,36,39,42,46) Of two siblings with renal
tubular acidosis and nephrocalcinosis, the sister exhibited
weakness and trembling, and developed active rickets at ten years
of age - manifestations of hypomagnesemia from the renal loss.
Suggestive of the possibility that they might have had magnesium
inadequacy from birth is the fact that their mother had a history
of seven spontaneous abortions;(33) magnesium deficiency is
contributory to abortions and preterm births.(4,8,9,11,12) A
young boy with renal magnesium wasting, associated with
aminoaciduria, glycosuria (at normal to low blood glucose
levels), growth retardation and osteoporosis, without intestinal
magnesium malabsorption, has been reported to respond well to
high dosage oral magnesium supplements;(33) most patients with
renal magnesium wasting have required parenteral magnesium
treatment.
Renal Damage from Calcemic Therapy of Magnesium-Low
Hypocalcemia - Tetany and convulsive seizures
characterize both hypomagnesemia and hypocalcemia. The usual
clinical pathological testing procedures disclose the calcium
deficit, whereas the magnesium deficit is more difficult to
detect,(49) an underlying magnesium inadequacy is apt to be
missed, and treatment is directed to correction of the
hypocalcemia. Since animal studies have shown that magnesium
deficient rats, on high calcium intakes, develop calcific lesions
in the corticomedullary area of the kidneys, and calcium
microliths in the loop and the ascending limb of the loop of
Henle (ALLH), the major site of magnesium reabsorption,(5,50-54)
it is possible that calcemic therapy, given to hypocalcemic
infants and children, who are magnesium deficient, might cause
renal tubular lesions in such areas. The laboratory findings, and
several clinical data support the premise that calcemic rather
than magnesium treatment of hypocalcemia with underlying
magnesium deficiency might play a role in long-lasting renal
magnesium leakage.
Two of the renal magnesium wasting young men, with
hypocalcemia, hypercalciuria and bilateral nephrocalcinosis, as
well as chondrocalcinosis, also malabsorbed magnesium.(37)
Another young man with chondrocalcinosis and renal magnesium
wasting had his disease first diagnosed at the age of six, when
hypocalcemic convulsions and hypokalemia failed to improve with
calcium and potassium treatment.(36) At that time, a renal biopsy
showed intraluminal tubular microliths in the renal
corticomedullary area; possibly the ALLH was involved. He, too,
was found to be a poor absorber of magnesium. The first reported
case, in 1944, of hypomagnesemic osteochondrosis was a six year
old boy who had been treated unsuccessfully with high dosage
vitamin D for neonatal hypocalcemic tetany and convulsions.(55)
His magnesium deficiency was not identified until he was six
months of age. Parenteral magnesium then controlled the
neuromuscular manifestations, but magnesium supplementation was
not sustained. Perhaps magnesium malabsorption caused his early
hypomagnesemic hypocalcemia; his later persistent magnesium
deficit might have been caused by renal damage-induced magnesium
wastage. Another young boy's renal magnesium wasting, that could
not be compensated for by oral or parenteral magnesium
supplements, developed osteochondritis, and later died of
CMP.(32) A child with a comparable history, but without
osteochondritis, died with hypertrophic CMP (at 13 years of
age).(48) Autopsy of a five month old baby girl, whose
convulsions had been unsuccessfully treated with high dose
vitamin D and intravenous calcium, and who was found to have
profound hypomagnesemia the last day of life,(56) disclosed renal
calcinosis, with calcium deposits in proximal tubules and Henle's
loop, as well as focal myocardial necrosis and calcium deposits,
and coronary arterial calcification. This case report seems
supportive of the premise that early calcemic therapy, in the
presence of magnesium deficiency (possibly caused by
malabsorption), can cause renal tubular damage that diminishes
renal magnesium reabsorptive capability. In view of the history
of infantile convulsions in four of her siblings, and death of
another sibling from cerebral arterial calcification at three
months, the metabolic disorder seems to be familial.
Patterns of Hereditary Renal Magnesium
Wasting - Three types of hereditary renal hypomagnesemia
have been suggested:(43) an autosomal dominant pattern of
inheritance is believed to be present in patients with isolated
familial hypomagnesemia; an autosomal recessive trait has been
suggested in familial hypomagnesemia caused by a low renal
magnesium threshold, that is associated with hypokalemia,
metabolic alkalosis, hypocalciuria and moderate salt wasting; and
familial hypomagnesemic hypercalciuria, caused by a defect in
absorption of both magnesium and calcium at the ALLH, with renal
calcinosis, also may be inherited as an autosomal recessive
trait. This condition has not been clearly separated from
hereditary distal renal tubular acidosis. A form of
hypomagnesemic hypokalemia that may be transmitted by an
autosomal recessive gene has been described in a young son of a
woman suffering from chronic hypomagnesemia(57). He had no
symptoms of his subnormal magnesium and potassium serum levels,
other than carpopedal spasms, that were not relieved by magnesium
or potassium therapy. This defect was postulated to be caused by
inability to maintain a normal gradient between intraand
extracellular magnesium and potassium.
Genetic Factors in Control of Magnesium Levels in
Plasma and Erythrocytes - Studies of identical and
fraternal twins have shown that red cell magnesium is
significantly more similar in monozygotic than in dizygotic
twins, and among family members than in unrelated
subjects.(21-23,58) Association of major histocompatibility
complex - human leukocyte antigens (HLA) alleles have been shown
to regulate red blood cell magnesium in humans; H-2 alleles are
involved in mice genetically selected for low red cell
magnesium.(22,23) HLA-B38 carriers, among blood donors, have
lower plasma and red cell magnesium levels than do other
genetically related groups.(23,59)
The latent tetany syndrome of magnesium deficiency(60) has
been associated with the HLA-B35 allele;(61); chondrocalcinosis,
which has been found in renal wasters of magnesium, has been
associated with the B15 antigen.(62) Type A behavior students,
many of whom were in the HLA-Bw35 group,(63,64) had a lowered red
cell level of Mg under stress, as well as lower plasma magnesium
levels. Interestingly, they had higher red cell zinc levels
(possibly linked with the GLO1 locus(63)), and excreted more
urinary zinc under stress.(21) Mitral valve prolapse, which has
also been associated with low magnesium levels, occurring more
frequently in patients with the latent tetany syndrome,(65-67)
also is associated with HLA-Bw35.(65-67) This allele is
associated with lower red cell Mg and much greater antibody
response to anti-influenza vaccination,(68) which might relate to
the role of magnesium in immunologic disorders, in which the
major histocompatability complex plays an important role.(69)
Neonatal Cardiovascular Damage - The early
arterial lesions, that were reported in 154 individual case
reports and in more than 500 infants reported in pathology
summaries of abnormalities found in stillborn infants and in
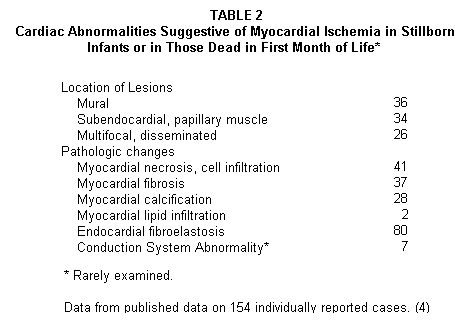
those dying in the first month of life Table 1(4,8))

include small vessel intimal fibroblastic proliferation,
elastica degeneration and calcification, changes such as have
been produced by experimental magnesium deficiency in laboratory
animals.(4-6) The coronary microcirculation was rarely examined
in the fetuses and infants tabulated in the 1980 report, but the
myocardial and endocardial lesions indicate probable damage to
the small arteries of the heart (Table 2(4-8)).
To correlate such changes with magnesium deficiency of the
fetus, which must reflect that of the mother, has to be
inferential, few data having been reported on the mothers of the
cited infants born with these or gross cardiovascular anomalies.
In the few reports indicating the maternal condition, spontaneous
abortions, toxemias, multiple births, frequent and/or multiple
pregnancies and diabetes, were all associated with poor outcomes
of pregnancy; in all magnesium inadequacy is
likely.(4,8,11,12,70-87) Also, prenatal magnesium supplements
have shown benefit; fewer toxemic pregnancies and low birth
weight infants among supplemented versus control mothers, have
been reported from extensive European retrospective,(88,89)
epidemiologic(90) and prospective double blind studies.(91,92)
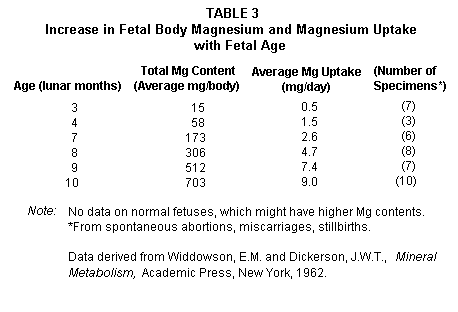
Since preterm infants have low magnesium levels, much of the
total fetal magnesium being accumulated in the last two lunar
months(4,8,93) (Table 3),
fetal inadequacy of magnesium seems to be a plausible
contributory factor to the higher incidence of cardiovascular
abnormalities in such infants, than in term singleton infants
born to mature normal non-multiparous mothers.
Maternal and Infant Cardiomyopathy -
Microangiopathy/CMP has been reported both in infants,(94-98) and
peripartally in mothers,(99-112) particularly with gestational
conditions associated with magnesium deficiency. A 1971 study
indicated that toxemic pregnancies were complicated by peripartum
CMP about six times as frequently as were normal pregnancies, and
that 7% of peripartal CMP occurred with twin pregnancies.(103)
The much greater vulnerability of marginally malnourished
multiparous and toxemic pregnant women to CMP, than of affluent
mothers, who usually are better nourished, was editorially
commented upon in 1968;(102) the condition is much rarer in the
developed world(108,112) than in Africa.(107,110)
Among the primary "idiopathic" cardiomyopathies, listed in a
1970(99) paper systematizing the many conditions associated with
CMP, were fibrotic CMP of infancy (suggested as possibly
attributable to hypercalcemia), familial myocardial fibrosis of
later childhood and adults, and CMP of pregnancy and the
puerperium, in each of which magnesium inadequacy may
participate. Magnesium deficiency or tissue loss also occurs in
several of the conditions that were listed as contributory to
secondary CMP: alcoholism, severe diarrhea, potassium deficiency
or excess, abnormal calcium deposition including that of
hyperparathyroidism, protein calorie malnutrition, beriberi (both
thiamin responsive and resistant, or catecholamine-toxicity.
Cited also were magnesium deficiency itself, and several
dysrhythmias.
Magnesium Deficiency and the Heart: There is growing
evidence that magnesium deficiency can contribute to a variety of
arrhythmias and that magnesium treatment is increasingly being
used for their management worldwide.(4,7,113-124) Although its
therapeutic use is based predominantly on its pharmacologic
effect, it may also be restoring a deficit.(117-121) Increased
intravascular coagulation, a probable factor in CMP,(99) might be
contributed to by a low ratio of magnesium to calcium.(12) And
finally, the myocardial lesions of experimental magnesium
deficiency in rats,(125-128) dogs,(129) and golden Syrian
hamsters(130-132) resemble those of CMP.
The familial occurrence of CMP was commented on in 1957, when
the term was first used to indicate myocardial disease without
major coronary arterial involvement.(133) Familial myocardial
fibrosis was listed as the commonest form of CMP in 1970;(99)
that year 13 families with hypertrophic CMP were reported(134)
and the following year 11 more such families were described, with
findings compatible with an autosomal dominant gene with high
penetrance.(135) The 1964(133) and 1970(99) reviews of genetic
diseases associated with cardiovascular abnormalities did not
include diabetes mellitus. However, in 1964 and 1967(137,138) the
minute focal areas of myocardial degeneration and fibrosis of
these diseases were attributed to medial necrosis of the small
intramural arteries and their occlusion by platelet aggregation,
which was then proposed as being partially explanatory of, not
only the rare genetic cardiomyopathies, but of that of alcoholics
and of juvenile diabetics; their similarity to that of
experimental magnesium deficiency(129) was noted.(138)
Diabetic Cardiomyopathy - Proliferative
lesions of the endothelium, that narrow or obliterate the lumina,
were described in diabetics' intramural small coronary arteries
in 1960,(139) Fibrotic CMP, stemming from microangiopathy of
diabetes, was described a decade later.(140) In 1972, it was
shown that 16 of 73 patients with diabetes mellitus had CMP;
autopsies in several showed mural coronary microangiopathy and
focal perivascular and interstital fibrosis of the endocardium
and subendocardium.(141)
Relationship with Magnesium Loss: It has been known
for almost half a century that poorly controlled insulin
dependent diabetes (Type I) causes magnesium loss.(142) An early
report also showed that those with juvenile diabetes are likely
to have the lowest serum magnesium values.(143) A study with
diabetic children, under strict medical and dietary control,
found that a third had hypomagnesemia (less than 1.4 mEq/L of
serum) as well as comparably low levels in red cells.(144) It
thus appears that diabetic children are more likely to be
magnesium deficient than are adult diabetics. As with adults, in
a study of 95 Type I children, those not optimally controlled had
the lowest serum magnesium values.145 This was verified in a
subsequent study of 63 such children, which further demonstrated
the deficit by retention of greater than 40% of a parenteral load
of magnesium.(146) In both of those clinical studies, increased
urinary magnesium output was noted, versus controls. Diabetic
gastro-enteropathy was suggested as a cause of magnesium
malabsorption that contributes to magnesium deficiency. Elevated
serum levels of low-density lipids and depressed levels of
high-density lipids, of poorly controlled diabetic children were
shifted to an improved pattern with better control that was
associated with higher serum magnesium.(144) This is in accord
with the demonstration that magnesium deficiency causes
substantial elevation of lowdensity and very low-density lipids
and decreased high-density lipids, accompanied by increased
platelet aggregation and thrombus formation, in rats fed a low
fat diet.(147,148) Since increased magnesium intake protected
against these effects, the authors suggested that the
dyslipidemia of diabetes mellitus might be mediated, at least in
part, by magnesium loss.(147,148)
Possible Relationship with Chromium: The dietary
intake of chromium is suboptimal in the American diet, its loss
is increased during pregnancy, and its deficiency has been linked
to maturity onset diabetes and arterial disease, and its
supplementation has increased high-density lipids
levels.(150-152) It has been suggested that, although the major
mineral abnormality in diabetes is that of magnesium, these
effects of chromium justify trial of its supplementation, as well
as that of magnesium, in juvenile diabetes.(153)
Congenital Deafness, Syncope, Arrhythmias, and Sudden
Death (Jervell-Lange-Nielsen Syndrome) - First reported
in 1957, as familial deaf-mutism with prolonged Q-T interval, CMP
and attacks of syncope, no abnormal blood electrolytes (calcium,
potassium and phosphorus) were then encountered.(154) In a 1964
paper on the obscure cardiomyopathies, involvement of the
nutrient arteries of the sinus node and atrio-ventricular node
was suggested to explain the high incidence of arrhythmias and
conduction abnormalities in this condition,(137) by the
investigator who later noted the similarity of the lesions of
human CMP to that produced by experimental magnesium deficiency
in dogs.(138) Among nine cases in six sibships, autopsies
disclosed focal hemorrhages near the atrioventricular and
sinoatrial nodes, with involvement of the left anterior
descending coronary artery (from which the nodal arteries arise),
and nodal fibrotic lesions.(155) Cochlear abnormalities (absent
stria vascularis and spiral ganglion of the ear) were speculated
to have been caused by fetal vascular damage. Since then, there
have been additional reports of the complete familial
syndrome,(156,157) of several members of a family with only sinus
node involvement,(158) and of a family in which arrhythmias
occurred alone or with deafness.(159)
Deafness Caused or Intensified by Experimental Magnesium
Deficiency: Weanling rats, that survived magnesium
deficiency that caused noise-induced convulsions and death in
litter mates, were found to have markedly decreased response to
sound,160 and to have cochlear damage (personal communication).
That the infantile impairment of hearing might have been caused
by magnesium deficiency is suggested by the significantly greater
hearing loss of magnesium deficient guinea pigs exposed to very
loud noise than did animals fed a magnesium rich diet, exposed to
the same noise.(161) Suggested mechanisms of the combined noise
plus magnesium deficiency-induced deafness were increased
catecholamine release, with constriction of the cochlear artery,
and decreased magnesium content of the fluid around the hair
cells, which allowed for their increased permeability, with
increased calcium and sodium influx and energy depletion in the
hair cells.(162,163)
Progressive Muscular Dystrophy, Marfan's Syndrome, and
Friedrich's Ataxia - Patients with the rare genetic
disorders: Marfan's syndrome and ataxia, or with the more common
Duchenne's muscular dystrophy are prone to CMP.(99,136-138)
Almost all of those with muscular dystrophy, which is inherited
as an X-linked trait, develop cardiac lesions,(164) which
resemble those of skeletal muscle.(165) It is of interest that a
patient with Duchenne's muscular dystrophy, who died suddenly,
had medial degeneration of the nutrient artery to the sinus node
and of the node, such as is seen in more generalized CMP, in
addition to scattered sites of myocardial necrosis.(165)
Electro-cardiographic study of 169 patients with this disease
disclosed abnormalities in 75%.(166)
Magnesium and Calcium in Duchenne's Muscular
Dystrophy: Markedly lower serum magnesium and higher serum
calcium levels in dystrophic patients than control values were
reported by one group of investigators,(167,168) but not by
another, who found that pre-adolescent patients had significantly
higher red blood cell magnesium than did postpubertal patients,
which was the reverse of findings in controls.(169) Fetuses at
risk of this disease, and a premature infant who later developed
typical Duchenne's disease, had 3-6-fold increased muscle calcium
and a lesser increase of muscle magnesium (18 to 57% above
normal); no necrotic fibers were detected.(170) Comparably
increased muscle calcium was seen in full blown dystrophy.(171)
The increase in their muscle calcium was considered a
non-specific part of the final common pathway leading toward
cellular degeneration and death.(170,171)
Among the data on the myocardium from studies with patients
with Duchenne's muscular dystrophy, although not directly
illustrative of the magnesium status, are a few that can be
correlated with animal models of hereditary muscular dystrophy
that provide information on magnesium (below). Abnormal
myocardial metabolism, suggestive of uncoupling of oxidative
phosphorylation (indicated by high inorganic phosphate, increased
glycolysis, and the high arteriovenous redox potential of
anaerobic metabolism) were detected in 11 Duchenne patients whose
blood samples were obtained by coronary sinus catherization.(172)
Six patients' biopsied muscle, not yet irreparably damaged by the
dystrophic disease, had excess catecholamine accumulation around
arterioles.(173)
Magnesium and Calcium, Myocardial Metabolism, and
Catecholamines in Models of Genetic Muscular Dystrophy: From
a strain of golden Syrian hamsters that had hereditary muscular
dystrophy, an inbred strain (BIO 14.6) was developed that
consistently developed spontaneous CMP.(174) The authors
commented that the lesions resembled those produced by excessive
catecholamines in rats and other species: augmentation of oxygen
consumption beyond requirements for cardiac work, and uncoupling
of oxidative phosphorylation. The earliest cardiac abnormality in
the CMP hamster, before myocardial necrosis developed, was
markedly decreased myocardial magnesium as compared with normal
hamsters (73 vs 115 mg/100 g dry weight), at 29 days of age.(175)
At two months, when the cardiac damage was moderate to severe in
the CMP strain, the myocardial Mg was the same (109 mg) as in the
normal hamsters of the same age. The increase in myocardial
calcium, over that of normals, however, was slight at the
prenecrotic phase (17 vs 12 mg), but marked at two months (215 vs
15 mg). Fed magnesium deficient diets, the CMP hamsters and
hybrids developed much more myocardial necrosis than did normal
hamsters fed the deficient diet. Adding MgCl2 (1.0mM/d) to the
low magnesium diet prevented myocardial necrosis of normal and
hybrid hamsters, but not of the CMP strain.(175) Comparable
myocardial mineral findings were reported in another study of
this CMP strain, that also provided ultramicroscopic evidence of
the progressive mitochondrial damage,(176) and in one that also
reported trace mineral findings: high zinc levels, possibly in
exchange for calcium.(177) The MDx mouse, which has a gene defect
at the locus homologous to the defective one in Duchenne's
muscular dystrophy,(178) also exhibited elevated myocardial
calcium at 10 days, 30 days and 254-347 days of life,(178) and at
5,10, and 23 weeks.(179) As in the BIO 14.6 hamsters,(175,176)
the magnesium changes were not notable. The energy metabolism
studies of skeletal muscle of the CMP hamster,(180-182) and of
myocardium of the MDx mouse,(179) yielded findings comparable to
those from Duchenne patients.(172,173) Uncoupling of oxidative
phosphorylation occurred in 50-80 day old CMP hamsters, the time
of most necrosis. Addition of 3mM MgCl2 to abnormal mitochondria
at the beginning of the experiment produced doubling of initial
rate of respiration and restored phosphorylative coupling to near
normal. This might bear on the lack of response to magnesium of
the hamsters with most advanced disease. Mitochondria from MDx
mice have decreased respiratory control, an observation also in
the dystrophic hamsters and in Duchenne's muscular dystrophy.
Cardiac norepinephrine studies,(183) prior to and during
development of congestive heart failure of the CMP hamster,
showed that its formation was most above normal in the
pre-necrotic phase, probably due to increased cardiac sympathetic
nerve activity. Similar but lesser increases in the myocardial
catecholamine were seen at the intermediate phase of cardiac
damage. During the final phase, when congestive heart failure had
developed, there was a markedly lower content possibly from a
"dilution" effect of hypertrophy and focal destruction of
adrenergic nerve terminals. Directly germane to the low
myocardial magnesium/calcium ratio in the pre-necrotic myocardium
of the CMP-hamster is the in vitro evidence that a low
magnesium/calcium ratio in adrenals(184) and peripheral
nerves(185) increases catecholamine secretion.
Vitamin E, Selenium, Zinc and Magnesium in Cardiomyopathy
of Muscular Dystrophy: Among the abnormalities produced by
experimentally induced vitamin E deficiency in several species is
necrotizing myopathy, that has pathologic changes resembling
those of human muscular dystrophy (which is not responsive to
vitamin E). In swine, the manifestations of tocopherol deficiency
include myocardial damage associated with fibrinoid necrosis of
the arteries.(186) Apart from the histopathologic similarities
between vitamin E deficiency-induced lesions, and those of
hereditary muscular dystrophy, the deficiency-altered muscles
also exhibit excessive oxygen utilization, possibly as a result
of uncoupling of oxidative phosphorylation, even before the
lesions are detectable.(186) Still another similarity is in the
lowered muscle magnesium content of both vitamin E deficient
dystrophic animals(187-189) and those with hereditary muscular
dystrophy before development of the histologic
lesions.(174-176)
Interrelationships between magnesium and vitamin E are
indicated also by precipitation of signs and lesions of magnesium
deficiency in vitamin E deficient normal rats,(190) and by
prevention of respiratory decline of (hepatic) mitochondria from
vitamin E deficient rats by administration of magnesium.(191)
Additionally, lipid peroxidation, which has long been known to be
counteracted by the anti-oxidant effect of vitamin E,(192) is
increased by magnesium deficiency in rat liver, muscle, heart and
other organs,(193,194) an effect suggesting that the magnesium
deficiency-induced myocardial damage might be mediated by free
radicals.(195) This premise has been substantiated by studies
with magnesium deficient golden Syrian hamsters (not the CMP
strain). Magnesium deficiency alone caused myocardial injury that
was protected against by vitamin E.(132) Intensification of the
myocardial damage by injection of the beta-catecholamine analog,
isoproterenol, was interpreted as showing impairment of tolerance
of oxidative stress by magnesium deficiency.(131,196) Direct
evidence that free radicals participate in the myocardial damage
caused by magnesium deficiency was provided by a study showing
that vitamin E deficiency increased the number and extent of the
lesions so induced and that its supplementation was
protective.(132) Combined vitamin E and magnesium protected
against (erythrocyte) membrane lipid peroxidation in magnesium
deficient Syrian hamsters.(196)
Selenium also protects against peroxidation of lipids, and its
deficiency (in China) causes CMP of Keshan disease.(197-199) Its
interactions with vitamin E are being considered, as they affect
muscle disease.(198-201) It has been found to spare vitamin E,
decreasing the amount required by the Syrian golden hamster
(after 120 days of E depletion), but it did not prevent the
myopathy.(202) A study of the muscle damage (determined by
increased release of creatine kinase) of vitamin E and
selenium-low calves when transferred from enclosures to open
pasture(203) recalls the intensification of neuromuscular signs
of magnesium deficiency of ruminants shifted from barns to
pasture,(204) which was attributed in part to the change from
warmth of the enclosure to stress of exposure to cold, with
catecholamine release.(205) The meaning of the increase in
intracellular zinc, that is associated with increased calcium in
the affected heart of dystrophic hamsters(177) is not clear. The
investigators hypothesized that zinc might be co-transported with
calcium across the cell membrane or substituted for calcium in
pathways affected by the high-energy ATP-pump. The role of zinc,
not as an antioxidant, but possibly through its ability to
stabilize membranes exposed to oxidative stress(206) might have a
protective function. Possibly pertinent zinc, magnesium, and
pyridoxine interrelationships are considered, below, under
homocystinuria.
Cystic Fibrosis and Cardiomyopathy; Interaction of
Nutrient Deficiencies? - Cystic fibrosis, the most
common lethal or semilethal genetic disease in the white
population, is inherited in an autosomal recessive manner.(207)
Loss of pancreatic enzyme activity is characteristic; complete
loss occurs in 80-85% of patients.(207,208) Malabsorption, the
degree of which depends on the extent of loss of pancreatic
function, leads to nutritional deficiencies of vitamin E and
other fat soluble vitamins; deficiencies of magnesium and
selenium - also implicated in myopathies - can also develop. In
about 10% of those with cystic fibrosis, CMP, characterized by
patchy focal areas of necrosis and fibrosis, complicates the
disease.(209) The lesions resemble those of "idiopathic" CMP,
human muscular dystrophy, and the CMP induced by experimental
vitamin E deficiency or magnesium deficiency.
Vitamin E, Magnesium, Calcium, and Selenium in
Cardiomyopathy of Cystic Fibrosis: The shortened red blood
cell survival of this disease responds to high dosage vitamin E
(10-fold higher than the normal recommended dietary
allowance,(209,210) but the muscle weakness (myopathy?) of cystic
fibrosis, is not responsive. In an early report(211) (additional
to those reviewed in 1988(209) of two brothers with cystic
fibrosis, who had steatorrhea that developed at six months of
age, one who died of pneumonia was found at autopsy to have not
only pancreatic damage, but also endomyocardial fibroelastosis.
In their discussion, the authors referred to eight additional
reports of myocardial damage in infants and young children with
pancreatic insufficiency of cystic fibrosis. Autopsy examination
of two additional patients, who had had hypercalcemia, disclosed
generalized arterial intimal proliferation and calcification and
renal calcification; one had mild rickets.(212) These
manifestations resemble those described above in patients with
renal wasting of magnesium, with and without magnesium
malabsorption.
Few data have been found on the magnesium status of cystic
fibrosis patients. A study of erythrocyte levels of magnesium,
calcium, zinc and sodium in eight children with cystic fibrosis,
and in four of the parents, showed that the patients had the
lowest magnesium, zinc and sodium values, and the highest calcium
levels; the parents had higher magnesium levels than did the
affected children, but lower than control adults values.(213)
There were higher magnesium and lower zinc contents of hair,
nails, and duodenal fluid from children with cystic
fibrosis;(214) the significance is unknown. They had very high
calcium content of their duodenal fluid. In this study the
magnesium level in sweat was slightly elevated; in another(215)
there was no difference between patients and normal children.
Neonatal hair from most of the 13 infants with cystic fibrosis
contained water soluble calcium versus less than 30% of controls,
and over ten times as much insoluble calcium as controls.(216)
There were similar findings with hair magnesium, but of lesser
magnitude. It was speculated that inability (of patients' hair)
to bind calcium and magnesium might be related to the basic
defect.
Tremor, nervousness, weakness and anorexia (symptoms of latent
tetany of magnesium deficiency(60)) developed in a young man with
cystic fibrosis complicated by cor pulmonale, who had recently
received furosemide (a loop diuretic that causes magnesium loss)
for heart failure, but only for several days.(217) When his serum
magnesium was found to be 1.4 mEq/L, and a magnesium load of 367
mg Mg disclosed urinary excretion of 612 mg in 48 hours, he was
treated with 4 g/d of magnesium for five days, with disappearance
of signs and symptoms of the deficiency. The renal excretion of
so much magnesium in the face of hypomagnesemia suggests renal
wasting. Acutely lowered serum magnesium developed in seven
cystic fibrosis patients with bowel obstruction from meconium
ileus, that had been treated with oral and rectal administration
of the mucolytic agent, N-acetylcysteine, and a hypertonic
solution of sodium diatrizoate.(218)
Considered above, under muscular dystrophy, are the data on
the CMP of selenium deficiency, and its interactions with vitamin
E.(197-203) A 1982 review of clinical data failed to support a
premise that selenium deficiency might be implicated in the
pathogenesis of cystic fibrosis.(198) However, since then, in a
discussion of the CMP complication, it was pointed out that
malabsorption with and without cystic fibrosis has caused low
plasma selenium levels.(201) A study with the golden Syrian
hamster showed that selenium prevented pancreatic atrophy induced
by vitamin E depletion.(219)
Homocystinuria and Cardiomyopathy; Interaction of
Nutrient Deficiencies? Pyridoxine-Dependence of
Homocystinurics: One of the conditions listed as being associated
with CMP is homocystinuria,(99) predominantly a vitamin
B6-dependent disorder.(220-222) There are several metabolic
abnormalities that give rise to homocystinuria, the most common
of which is a Mendelian recessive trait that causes deficient
activity of cystathionine beta-synthase, an enzyme that contains
pyridoxal phosphate.(220)Almost half of the patients respond to
very high dosage pyridoxine (up to 300 times more pyridoxine than
is needed for correction of a simple deficiency; they may have
slight (residual) activity of this enzyme.(220-221) Those with a
more complete deficiency of the enzyme, or with a metabolic block
after formation of cystathionine also require additional dietary
modification and/or supplementation.
Among the pathologic changes in homocystinuric patients are
several germane to development of CMP: the occurrence of thrombi,
not only in large arteries but in the microvasculature, with
fraying of the elastica and premature arteriosclerosis.(223) CMP
is not frequently reported, but Marfan's syndrome, another
condition associated with CMP,(99,137) has occurred in
homocystinuric patients.(220,223)
Additional Nutritional Treatment of Homocystinuria: Folate
B12, Amino Acid Intake Modification and Possibly Magnesium and
Zinc: Patients with deficiency of cystathionine synthase,
accompanied by abnormally low serum folate levels have the folate
level further lowered by pyridoxine treatment, and folate
repletion is necessary for the chemical response to
pyridoxine.(220,224) Vitamin B12 supplements are necessary for
those whose metabolic block is after formation of cystathionine.
Those with methionine accumulation also require methionine
restriction and cysteine or choline supplements. Perhaps vitamin
B6 treatment from infancy might be effective.(222)
Pyridoxal phosphate, which is necessary for activity of
cystathionine synthase, requires magnesium as a cofactor for this
and for many other of its enzymatic reactions.(225) Studies in
the 1960s(226-229) and more recently(230-233) have shown that
vitamin B6 is necessary for the maintenance of magnesium and zinc
tissue levels, and that the pyridoxine-dependent enzymes also
require these minerals. Thus, adding magnesium and zinc to
vitamin B6 supplementation of patients with unduly high vitamin
B6 requirements seems worthy of trial.
Presented here is a postulate that magnesium deficiency caused
by genetic variations in magnesium metabolism, in conjunction
with marginal magnesium intake, is a contributory factor to
gestational complications, including perinatal and neonatal CMP.
Severe forms of familial magnesium deficiency: isolated
malabsorption and renal wasting of magnesium have been
identified. The possibility that the underlying inherited
abnormality in the renal magnesium wasting syndromes is that of
magnesium malabsorption is presented. It is suggested that renal
magnesium wasting may be caused by damage at the major site of
tubular magnesium reabsorption, when infants with hypomagnesemic
hypocalcemia are provided calcemic treatment without magnesium
repletion. It is proposed that there may be degrees of magnesium
malabsorption, and that there are genetic differences in plasma
and cellular magnesium levels in different ethnic groups; low
values have been associated with specific HLA groups. These
differences might be part of the metabolic basis of other
inherited diseases.
The heritable diseases, that are complicated by cardiovascular
damage, especially microangiopathy leading to generalized or
nodal CMP, are of particular interest as regards the possibility
of contributory magnesium inadequacy, because experimental
magnesium deficiency causes similar arterial and myocardial
lesions. There is direct evidence of abnormalities in magnesium
retention and/or tissue levels in diabetes mellitus, especially
in the juvenile form, in which microangiopathy causes serious
early complications. Since chromium, like magnesium, participates
in carbohydrate and lipid metabolism, and it seems to be linked
to maturity onset diabetes, its role, with and without magnesium
in pregnant diabetic women, and in infants born to such women
might be worth exploring.
The familial syndrome of congenital deafness, syncope,
arrhythmias, and sudden death has not been correlated with
magnesium abnormality in the literature, but it bears similarity
to manifestations produced by magnesium deficiency. The
myocardial lesions involving the conducting tissue and nodes
differ from those of the more commonly described
cardiomyopathies, and those of magnesium deficiency, only in
their location. The deafness has been presumed to be caused by
cochlear damage, caused by damage of the nutrient arteries to the
inner ear; it can be correlated with the hearing loss of
magnesium deficient weanling rats and of noise-exposed magnesium
deficient guinea pigs. Might magnesium supplementation during
pregnancy of diabetics or of members of families with risk of the
Jervell-Nielsen-Lange syndrome protect the infant, and might it
slow or limit progression of the disease manifestations in those
afflicted?
The skeletal muscle and myocardial lesions of Duchenne
progressive muscular dystrophy, have been compared with those
produced by magnesium deficiency, and are similar to those seen
in models of genetic muscular dystrophy, which also have
comparable metabolic findings. CMP hamsters were shown to lose
skeletal and myocardial muscle magnesium and to gain large
amounts of calcium before the necrosis developed, even when not
fed a magnesium deficient diet. They also exhibited high
myocardial catecholamine content and uncoupling of oxidative
phosphorylation, abnormalities also seen in the human disease.
Magnesium supplementation delayed, but did not prevent the damage
in the CMP hamster, but prevented it in hybrid and normal
magnesium deficient hamsters. The lesions of the human disease
also resemble those of vitamin E deficient animals, which also
have low muscle magnesium content. The CMP induced by magnesium
deficiency in golden Syrian hamsters has been shown to be
intensified by vitamin E deficiency and reduced by its
supplementation. The capacity of magnesium deficient Syrian
hamsters to withstand the oxidative stress of catecholamine
challenge was diminished without prior supplementation with
vitamin E, indicating free radical participation in magnesium
deficiency-induced CMP. Treatment with high dosage vitamin E has
not influenced Duchenne muscular dystrophy. Perhaps treatment
with both vitamin E and magnesium might delay its
progression.
The shortened erythrocyte survival time of mucoviscidosis or
cystic fibrosis requires ten times the normal intake of vitamin E
for correction; the CMP that develops in about 10% of the cases
is unresponsive. The vitamin E deficiency is attributed to
malabsorption, from steatorrhea resulting from loss of pancreatic
enzymes that can be complete in up to 85% of the patients with
cystic fibrosis. It can be presumed that this condition also
interferes with absorption of other nutrients, including
magnesium. Few data have been found on the magnesium status,
other than magnesium deficiency in several patients under
short-term diuretic treatment or other therapy not usually
associated with magnesium depletion, for complications of
mucoviscidosis. However, these few data suggest that these
patients may be unduly vulnerable to magnesium loss. The
interrelationships of magnesium with vitamin E, additional to
those discussed in relation to Duchenne disease, are indicated by
evidence that vitamin E deficiency has precipitated magnesium
deficiency in rats, and magnesium has prevented vitamin E-induced
respiratory decline and lipid peroxidation. Might vitamin E plus
magnesium supplements prove helpful in management of cystic
fibrosis? The observation that selenium prevented the pancreatic
atrophy induced by vitamin E depletion in the golden hamster,
might be worthy of exploration in humans.
Almost half of homocystinuric patients (those with some
cystathionine synthase activity) require very high dosage
pyridoxine (up to 300 times normal) for correction of the
biochemical abnormality. The supplements or dietary restriction
required by patients who have a more complete deficiency of the
enzyme, or with a metabolic block after formation of
cystathionine can include vitamin B12, folate, methionine
restriction, and cysteine supplements. Progression of the disease
despite the several nutritional approaches, has suggested that
(pyridoxine) supplementation be instituted in infancy. CMP has
not been reported in homocystinuria, but patients are subject to
microvascular thrombosis, which can contribute to CMP, as well as
to large artery thromboses. Homocystinuria has been reported in
patients with Marfan's syndrome (associated with CMP), and
premature arteriosclerosis with frayed elastica and medial
degeneration is common. Both because the arteriopathy (and
hypercoagulability) resemble that produced by magnesium
deficiency, and because pyridoxal phosphate, which is necessary
for activity of cystathionine synthase, requires magnesium as a
cofactor, and is necessary for maintenance of tissue levels of
magnesium, addition of magnesium to the therapeutic regimen
deserves trial. Since tissue levels of zinc are also dependent on
adequate pyridoxine, the effect of addition of zinc is worth
determining.
The major emphasis in this paper has been on genetic disorders
that are associated with CMP, to which magnesium deficiency,
caused by higher than average magnesium requirements, might be
contributory. Magnesium is protective against arterial and
myocardial damage, and interacts with vitamins E and B6,
dependencies or deficiencies of which have been implicated in the
genetic diseases complicated by CMP. A few data have been
presented on the trace minerals: selenium, chromium, and zinc
that may also bear on these diseases.
1. M.S. Seelig. The requirement of magnesium by the normal
adult. Am. J. Clin. Nutr. 14, 342-390, (1964).
2. M.S. Seelig. Magnesium requirements in human nutrition.
Magnesium Bull. 3 (1a), 26-47 (1981).
3. M.S. Seelig. Nutritional status and requirements of
magnesium, with consideration of individual differences and
prevention of cardiovascular disease. Magnesium Bull. 8, 170-185
(1986).
4. M.S. Seelig. Magnesium Deficiency in the Pathogenesis of
Disease. Early Roots of Cardiovascular, Skeletal and Renal
Abnormalities. (L.V. Avioli, Ed.) Publ Plenum Medical Book Co.
New York, N.Y. 1980.
5. M.S. Seelig and H.A.Heggtveit. Magnesium interrelationships
in ischemic heart disease: A review. Am. J. Clin. Nutr. 27, 59-79
(1974).
6. M.S. Seelig and F.J. Haddy. Magnesium and the Arteries: I.
Effects of magnesium deficiency on arteries and on retention of
sodium, potassium, and calcium. In Magnesium in Health and
Disease (M. Cantin, M.S. Seelig, Eds.) Spectrum, N.Y, 1980. (2nd
Intl. Mg Sympos. Quebec, 1976) pp 605-638.
7. M.S. Seelig. Cardiovascular consequences of magnesium
deficiency and loss: pathogenesis, prevalence and manifestations-
magnesium and chloride loss in refractory potassium repletion.
Am. J. Cardiol. 63, 4G-21G (1989).
8. M.S. Seelig. Early nutritional roots of cardiovascular
disease. In Nutrition and Heart Disease (H.K.Naito, Ed.) Proc.
19th Ann. Mtg. Am. Coll. Nutr. 1978), SP Medical & Sci Books,
New York, 31-59 (1982).
9. M.S. Seelig. Prenatal and neonatal mineral deficiencies:
magnesium, zinc and chromium. In Clinical Disorders in Pediatric
Nutrition. (F. Lifshitz, Ed.) Marcel Dekker, N.Y., N.Y., 1982,
pp. 167-196.
10. M.S. Seelig. Nutritional roots of combined system
disorders. In Clinical Disorders in Pediatric Nutrition. (F.
Lifshitz, Ed.) Marcel Dekker, N.Y., N.Y., 1982, pp. 327-351.
11. Seelig, M.S. Magnesium in pregnancy: special needs for the
adolescent mother. J. Am. Coll. Nutr. 10, 566 (1991).
12. Seelig, M.S. Interrelationship of magnesium and estrogen
in cardiovascular and bone disorders, eclampsia, migraine and
premenstrual syndrome. J. Am. Coll. Nutr. 12 (1993) (in
press).
13. L. Paunier and I.C.Radde. Normal and abnormal magnesium
metabolism. Bull. Hosp. Sick Childr.(Toronto) 14, 16-23
(1965).
14 J. Salet, C. Polonovski, F. DeGouyon, G. Pean, B. Melekian
and J.P. Fournet. (Hypocalcemic tetany deriving from congenital
hypomagnesemia. A new metabolic disease.) Arch. Franc. Pediat.
23, 749-768 (1966). (in French)
15. J. Salet, C. Polonovski, J.P. Fournet, F. DeGouyon, P.
Aymard, G. Pean and J.L. Taillemite. (Demonstration of the
familial nature of hypomagnesemia.) Arch. Franc. Pediat. 27,
550-551 (1970). (in French)
16. D. Skyberg, J.H. Stromme, R. Nesbakken and K. Harnaes.
Neonatal hypomagnesemia with a selective malabsorption of
magnesium--A clinical entity. Scand. J. Clin. Investig. 21,
355-363 (1968).
17. J.H. Stromme, R. Nesbakken, T. Normann, F. Skjorten and B.
Johannessen. Familial hypomagnesemia. Biochemical, histological
and hereditary aspects studied in two brothers. Acta Paediat.
Scand. 58, 433-444 (1969).
18. H.J. Gitelman, J.B. Graham and L.G. Welt. A new familial
disorder characterized by hypokalemia and hypomagnesemia. Trans.
Assoc. Amer. Physicians 79, 221-235 (1966).
19. R.M. Freeman and E. Pearson. Hypomagnesemia of unknown
etiology. Am. J. Med. 41, 645-656 (1966).
20. L. Sann, P. Moreau, B. Longin, J. Sassard and R. Francois.
(Bartter's syndrome, associated with hypercorticism, phosphorus
and magnesium diabetes and familial tubulopathy. Arch. Franc.
Pediat. 32, 349-366 (1975). (in French)
21. J.G. Henrotte, P.F Plouin, C. Levy-Leboyer, G. Moser, N.
Sidoroff-Girault. G. Franck, M. Santarromana and M. Pineau. Blood
and urinary magnesium, zinc, calcium, free fatty acids, and
catecholamines in type A and type B subjects. J. Am. Coll. Nutr.
4, 165-172 (1985).
22. J.G. Henrotte. Genetic regulation of blood and tissue
magnesium content in mammals. Magnesium 7, 306-314 (1988).
23. J.G. Henrotte, M. Pla and J. Dausset. HLA- and
H-2-associated variations of intra- and extracellular magnesium
content. Proc. Natl. Acad. Sci. USA 87, 1894-1898 (1990).
24. W.E.C. Wacker. Magnesium and Man. Harvard Univ Press,
Cambridge, MA, 1980.
25. B.L. Vallee. Metal and enzyme interactions: correlation of
composition, function, and structure. In The Enzymes 3, 225-270
(1960).
26. C.C. Booth, M.B. Babouris, S. Hanna, and I. MacIntyre.
Incidence of hypomagnesemia in intestinal malabsorption. Brit. J.
Med. 2:141-143 (1963).
27. L. Paunier, I.C. Radde, S.W. Kooh, P.E.E. Conen and D.
Fraser. Primary hypomagnesemia with secondary hypocalcemia in an
infant. Pediatrics 41, 385-402 (1968).
28. L. Paunier. Magnesium malabsorption. Adv. Intern. Med.
Pediat. 42, 113- 131, (1979).
29. M. Friedman, G. Hatcher and L. Watson. Primary
hypomagnesemia with secondary hypocalcemia in an infant. Lancet
1, 703-705 (1967).
30. S. Nordio, A. Donath, F. Macagno and R. Gatti. Chronic
hypomagnesemia with magnesium dependent hypocalcemia I. A new
syndrome with intestinal malabsorption. II. Magnesium, calcium
and strontium. Acta Paediat. Scand. 60, 441-448, 449-455
(1971).
31. N.H. Main, R.J. Morgan, R.I. Russell, M. Hall, J.F.
Mackenzie, A. Shenkin and G.S. Fell. Magnesium deficiency in
chronic inflammatory bowel disease and requirements during
intravenous nutrition. J.P.E.N. 5, 15-19 (1981).
32. W.G. Klingberg. Idiopathic hypomagnesemia and
osteochondritis. Ped. Res. 4, 452, (1970); Death from
cardiomyopathy (personal communication).
33. M.F. Michelis, A.L. Drash, L.G. Linarelli, F.R. DeRubertis
and B.B.Davis. Decreased bicarbonate threshold and renal
magnesium wasting in a sibship with distal renal tubular
acidosis. Metabolism 21, 905-920 (1972).
34. J.W. Mace, K.M. Hambidge, R.W. Gotlin, R.S. Dubois, C.S.
Solomons and F.H. Katz. Magnesium supplementation in Bartter's
syndrome. Arch. Dis. Child. 48, 485-487 (1973).
35. B.E. Booth, A and Johanson. Hypomagnesemia due to renal
tubular defect in reabsorption of magnesium. J. Pediat. 84,
350-354, (1974).
36. L. Runeberg, Y. Collan, E.J. Jokinen, J. Lahdevirta and A.
Aro. Hypomagnesemia due to renal disease of unknown origin. Am.
J. Med. 59, 873-882 (1975).
37. A. Rapado A. and J.M. Castrillo. Chondrocalcinosis and
hypomagnesemia; Nephrocalcinosis and hypomagnesemia. In Magnesium
in Health and Disease (2nd Intl Sympos on Magnesium, Quebec,
1976; M. Cantin, M.S. Seelig, Eds.) Spectrum, New York, 1980. pp
355-364; 485-497.
38. M.S. Seelig, A.R. Berger, L.A. Avioli. Speculations on
renal, hormonal, and metabolic aberrations in a patient with
marginal magnesium deficiency. In Magnesium in Health and Disease
(2nd Intl Sympos on Magnesium, Quebec, 1976; M. Cantin, M.S.
Seelig, Eds.) Spectrum, New York, 1980. pp 459-468.
39. F. Manz, A. Anders, P. Janka, I. Lombeck and K. Scharer.
(Renal magnesium wasting, incomplete tubular acidosis,
hypercalciuria and nephrocalcinosis in 4 children and adults.)
Magnesium Bull. 1, 151 (1979). (in German)
40. F.M. Bauer, P. Glasson, M.B. Valloton and B. Courvoisier.
(Bartter's syndrome, chondrocalcinosis, and hypomagnesemia.
Schweiz. med. Wschr. 109, 1251-1256 (1979). (in German)
41. H.G. Guellner, J.R. Gill, F.C. Bartter, Correction of
hypokalemia by magnesium repletion in familial hypokalemic
alkalosis with tubulopathy. Am. J. Med.71, 578-582 (1981).
42. M.A. Mayoux-Benhamou, D. Clerc, D. Ganeval, N. Pertuisset
and P. Massias. [Articular chondrocalcinosis and hypomagnesemia
of renal origin; 2 cases] Rev. Rhum. Mal. Ostoartic. 52, 545-548
(1985).
43. J. Rodriguez-Soriano, A. Vallo and M. Garcia-Fuentes.
Hypomagnesaemia of hereditary renal origin. Pediat. Neprol. 1,
465-72 (1987).
44. M.G. Bianchetti, E. Girardin, P.C.C. Sizonenko and L.
Paunier. Metabolic studies on a new case of
hypomagnesaemia-hypokalaemia. Magnesium Res. 1, 116 (1988).
45. E. Pronicka and B. Gruszczynska. Familial hypomagnesaemia
with secondary hypocalcaemia--autosomal or X-linked inheritance?
J. Inhert. Metab. Dis. 14, 397-399 (1991).
46. J.P. de-Filippi, P.P. Diderich and J.M. Wouters.
[Hypomagnesemia and chondrocalcinosis.] Ned. Tijdscgr. Geneesk.
136, 139-141 (1992). (in Dutch)
47. L.E. Sutherland, P. Hartroft, J.W. Balis, J.D. Bailey and
M.J. Lynch. Bartter's syndrome. A report of four cases, including
three in one sibship, with comparative histologic evaluation of
the juxtaglomerular apparatuses and glomeruli. Acta Paediat.
Scand. Suppl. 201, 1-25 (1970).
48. J.E. Riggs, W.G. Klingberg, E.B. Flink, S.S.Schochet Jr.,
A.A. Balian and J.J. Jenkins 3d. Cardioskeletal mitochondrial
myopathy associated with chronic magnesium deficiency. Neurology.
42, 128-130 (1992).
49. R.J. Elin. Assessment of magnesium status. In Magnesium in
Health and Disease (Y. Itokawa, J. Durlach Eds.) J. Libbey,
London, 1989 (Fifth Intl Mg Sympos, Kyoto, 1988), pp.
137-146.
50. R. Hess, I. MacIntyre, N. Alcock and A.G.E. Pease.
Histochemical changes in rat kidney in magnesium deprivation.
Brit. J. Exp. Path. 40, 80-86 (1959).
51. L.G. Welt. Experimental magnesium depletion. Yale J. Biol.
Med. 36, 325- 349 (1964).
52. J. Oliver, M. MacDowell and R. Whang. The renal lesion of
electrolyte imbalance. IV. The intranephric calculosis of
experimental magnesium depletion. J. Exp. Med. 124, 263-299
(1966).
53. G.A. Quamme. Renal handling of magnesium: Drug and hormone
interactions. Magnesium 5, 248-272 (1986).
54. M.S. Seelig. Calcium and magnesium deposits in disease, In
Handbook on Metal-Ligand Interactions in Biological Fluids (G.
Berthon, Ed.) Marcel Dekker, Inc. New York, 1993 (in press)
55. J.F. Miller. Tetany due to deficiency in magnesium. Its
occurrence in a child of six years with associated
osteochondrosis of capital epiphysis of femur. J. Dis. Child. 67,
117-119 (1944).
56. M. Vainsel, G. Vandervelde, J. Smulders, M. Vosters, P.
Nubain and H. Loeb. Tetany due to hypomagnesemia with secondary
hypocalcemia. Arch. Dis. Childh. 45, 254-258, (1970).
57. L. Paunier and P.C. Sizonenko. Asymptomatic chronic
hypomagnesemia and hypokalemia in a child: cell membrane disease.
J. Pediat. 88, 51-55 (1976).
58. P. Darlu and J.G. Henrotte. The importance of genetic and
constitutional factors in human red blood cell magnesium control.
In Magnesium in Health and Disease (M. Cantin, M.S. Seelig, Eds.)
Spectrum, N.Y, 1980. (2nd Intl. Mg Sympos. Quebec, 1976) pp
921-927.
59. J.G. Henrotte. Relationship between red blood cell
magnesium and HLA antigens. Tissue Antigens 15, 419-439
(1980).
60. J. Durlach. Magnesium in Clinical Practice. (Translated by
D. Wilson). John Libbey & Co. Ltd. London, 1985.
61. B. Maertens de Noordhout, J.G. Henrotte and P.
Franchimont. Latent tetany, magnesium and HLA tissue antigens.
Magnesium Bull. 9, 118-121 (1987).
62. M. Megard, E. Andre-Fouet, E. Guisti, H. Betuel and L.
Gebhurer. Articular chondrocalcinosis, Association with antigen
B15. Presse Med 13, 1727 (1984). (in French)
63. P. Darlu, E. Defrise-Gussenhoven, Y. Michotte, C. Susanne
and J.G. Henrotte. Possible linkage relationship between genetic
markers and blood magnesium and zinc. A twin study. Acta Genet.
Med. Gemell. Roma 34, 109-112 (1985).
64. J.G. Henrotte. Genetic regulation of red blood cell
magnesium content and major histocompatibility complex. Magnesium
1, 69-80, 1982.
65. J. Durlach J, J.G. Henrotte, V. Lepage, A. Elchidial and
L. Degos. HLA-Bw35 antigen, mitral valve prolapse and blood
magnesium level. In Magnesium Deficiency. Physiopathology and
Treatment Implications. M.J. Halpern and J. Durlach, Eds.) First
Europ. Mg Congr. Lisbon 1983 (Karger, Basel 1985) pp. 95-101.
66. L.D. Galland, S,M. Baker and R.K. McLellan. Magnesium
deficiency in the pathogenesis of mitral valve prolapse.
Magnesium 5, 165-174 (1986).
67. J. Durlach and V. Durlach. Idiopathic mitral valve
prolapse and magnesium. State of the art. Magnesium Bull. 8,
156-169 (1986).
68. J.G. Henrotte, C. Hannoun, A. Benech and J. Dausset.
Relationship between postvaccinal anti-influenza antibodies,
blood magnesium levels, and HLA antigens. Hum. Immunol. 12, 1-8
(1985).
69. J.G. Henrotte. Recent advances on genetic factors
regulating blood and tissue magnesium concentrations.
Relationships with stress and immunity. In Magnesium in Health
and Disease (Y. Itokawa, J. Durlach Eds.) J. Libbey, London, 1989
(Fifth Intl Mg Sympos, Kyoto, 1988), pp. 285-289.
70. V. Kontopoulos, M.S. Seelig, J. Dolan, A.R. Berger and
R.S.Ross. Influence of parenteral administration of magnesium
sulfate to normal pregnant and to pre-eclamptic women. In
Magnesium in Health and Disease (M. Cantin and M.S. Seelig, Eds.)
Spectrum, N.Y, 1980. (2nd Intl. Mg Sympos. Quebec, 1976) pp
839-848.
71. Weaver K. A possible anticoagulant effect of magnesium in
pre-eclampsia. In Magnesium in Health and Disease (M. Cantin and
M.S. Seelig, Eds.) Spectrum, N.Y, 1980. (2nd Intl. Mg Sympos.
Quebec, 1976) pp 833-838.
72. K.B. Franz. Correlation of urinary magnesium excretion
with blood pressure of pregnancy. Magnesium Bull. 4, 73-78
(1982).
73. K. Weaver. Pregnancy-induced hypertension and low birth
weight in magnesium deficient ewes. Magnesium 5, 191-200
(1986).
74. G. Ajayi. Serum magnesium concentration in premenopausal,
menopausal women, during normal and EPH-gestosis pregnancy and
the effect of diuretic therapy in EPH-gestosis. Magnesium Bull.
10, 72-76 (1988).
75. G.P. Palla, P. Giaquinto, P.R. Moro, E. Maniccia, G.
Carelli and S. Mancuso. Magnesium load test in pregnancy
hypertension. Clin. Exp. Hypertens. Pregn. B7, 159-163
(1988).
76. A. Sjogren, G. Gennser and P. Rymark. Reduced
concentrations of magnesium, potassium and zinc in skeletal
muscle from women during normal pregnancy or eclampsia. J. Am.
Coll. Nutr. 7, 408 (1988).
77. A. Wynn and M. Wynn M. Magnesium and other nutrient
deficiencies as possible causes of hypertension and low
birthweight. Nutr. Health 6, 69-88 (1988).
78. E.B. Dawson and R. Kelly. Calcium, magnesium and lead
interrelationships in preeclampsia. J. Am. Clin. Nutr. 51, 512
(1990).
79. L. Wibell, M. Gebre-Medhin and G. Lindmark. Magnesium and
zinc in diabetic pregnancy. Acta Pediat. Scand. Suppl. 320,
100-106 (1985).
80. F. Mimouni, M. Miodovnik, R.C. Tsang, J. Holroyde, P.S.
Dignan and T.A. Siddiqi. Decreased maternal serum magnesium
concentration and adverse fetal outcome in insulin-dependent
diabetic women. Obstet. Gynecol. 70:85-88, 1987.
81. M. Miodovnik, F. Mimouni, T.A. Siddiqi and R.C. Tsang.
Periconceptional metabolic status and risk for spontaneous
abortion in insulin-dependent diabetic pregnancies. Am. J.
Perinatol. 5, 368-373 (1988).
82. G.P. Palla, F. Castaldo, P.R. Moro, P. Giaquinto, G.
Carelli, A. Caruso, A. Lanzone, S. Mancuso. Intravenous magnesium
load test in normal and diabetic pregnant women. Magnesium Res.
2:91-92, 1989. Proc. Fifth Intl Mg Sympos., Kyoto, Japan, August
8-12, 1988.
83. M.F. Greene, J.W. Hare, M. Krache, M. Phillippe, V.A.
Barss, D.H. Saltzman, A. Nadel, M.D. Younger, L. Heffner and J.E.
Scherl. Prematurity among insulin-requiring diabetic gravid
women. Am. J. Obstet. Gynecol. 161, 106-111 (1989).
84. P.R. Garner, M.E. D'Alton, D,K. Dudley, P. Huard, and M.
Hardie. Preeclampsia in diabetic pregnancies. Am. J. Obstet.
Gynecol. 163, 505-508, 1990.
85. C.K. Lin, P.L. Kuo, H.C. Liu, K.I. Yau, H.S. Chang, T.R
Wang and S.H. Chen. Clinical analysis of infants of diabetic
mothers. Acta Paediat. Sin. 30, 233-239 (1989).
86. A.Y. Ranade, R.H. Merchant, R.T. Bajaj and N.C. Joshi.
Infants of diabetic mothers--an analysis of 50 cases. Indian
Pediat. 26, 366-370 (1989).
87. M.S. Djurhuus, N.A. Klitgaard and H. Beck-Nielsen.
[Magnesium deficiency and development of late diabetic
complications] Ugeskr. Laeger. 153, 2108-2110 (1991). (in
Danish)
88. A. Conradt A, H. Weidinger. [The central position of
magnesium in the management of fetal hypotrophy - a contribution
to the pathomechanism of utero-placental insufficiency,
prematurity and poor intrauterine fetal growth as well as
pre-eclampsia.] Magnesium Bull. 4, 103-124 (1982).
89. A. Conradt, H. Weidinger H, H. Algayer. Magnesium therapy
decreased the rate of intrauterine fetal retardation, premature
rupture of membranes and premature delivery in risk pregnancies
treated with betamimetics. Magnesium 4, 20-28 (1985).
90. V. Kuti, M. Balazs, F. Morvay, Z. Varenka, A. Szekly, M.
Szucs. Effect of maternal magnesium supply on spontaneous
abortion and premature birth and on intrauterine foetal
development: experimental epidemiological study. Magnesium Bull.
3:73-79 (1981).
91. L. Spaetling, G. Spaetling. Magnesium supplementation in
pregnancy. A double-blind study. Brit. J. Obstet. Gynec. 95,
120-125 (1988).
92. L. Kovacs, B.G. Molnar, E. Huhn, L. Bodis. [Magnesium
substitution in pregnancy. A prospective, randomized double-blind
study]. Geburtsch. Frauenheil. 48, 595-600, 1988. (in German)
93. E.M. Widdowson and J.W.T. Dickerson. Chemical composition
of the body. In Mineral Metabolism. 2: Part A (C.L. Comar and F.
Bronner, Eds.) Academic Press, New York, 1962, pp. 2-247.
94. W.H. Haese, B.J. Maron, M. Mirowski, R.D. Rowe, G.M.
Hutchins. Peculiar focal myocardial degeneration and fatal
ventricular arrhythmias in a child. New Engl. J. Med. 287,
180-181 (1972).
95. J.E. Ferguson II, K.S. Harney and J.A.Bachicha. Peripartum
maternal cardiomyopathy with idiopathic cardiomyopathy in the
offspring. A case report. J. Reprod. Med. 31, 1109-1112
(1986).
96. R.G. Weintraub, M.J. Swinburn and L. Lee. Neonatal
hypertrophic cardiomyopathy: a case report and family study.
Austral. Paediat. J. 23, 249-251 (1987).
97. M.D. Reller and S. Kaplan. Hypertrophic cardiomyopathy in
infants of diabetic mothers: an update. Am. J. Perinatol. 5,
353-358 (1988).
98. J.N. McMahon, P.J. Berry and H.S. Joffe. Fatal
hypertrophic cardiomyopathy in an infant of a diabetic mother.
Pediat. Cardiol. 11, 211-212 (1990).
99. R.E.B. Hudson. The cardiomyopathies: order from chaos. Am.
J. Cardiol. 25, 70-77 (1970).
100. J.B. Johnson., G.H. Mir, P. Flores and M. Mann.
ldiopathic heart disease associated with pregnancy and the
puerperium. Am. Heart J. 72, 809-816 (1966).
101. T.A.D. Govan. Myocardial lesions in fatal eclampsia.
Scot. Med. J. 2, 187-192 (1966).
102. Unsigned Editorial. Cardiomyopathy and pregnancy. Brit.
J. Med. 4,269-270 (1968).
103. J.G. Demakis and S.H. Rahimtoola. Peripartum
cardiomyopathy. Circulation. 44, 964-968 (1971).
104. D.G. Julian and P. Szekely. Peripartum cardiomyopathy.
Prog. Cardiovasc. Dis. 27, 223-240 (1985).
105. A.K. Adler and M.R. Davis. Peripartum cardiomyopathy: two
case reports and a review. Obstet. Gynecol. Surv. 41, 675-682
(1986).
106. D.C. Homans. Peripartum cardiomyopathy. New Engl. J. Med.
312, 1432-1437 (1985).
107. A.O. Falase. Peripartum heart disease. Heart Vessels
Suppl 1, 232-235 (1985).
108. F.G. Cunningham, J.A. Pritchard, G.D. Hankins, P.L.
Anderson, M.J. Lucas and K.F. Armstrong. Peripartum heart
failure: idiopathic cardiomyopathy or compounding cardiovascular
events? Obstet. Gtnecil. 67, 157-168 (1986).
109. J.B. O'Connell, M.R. Costanzo-Nordin, R. Subramanian,
J.A. Robinson, D.E. Wallis, P.J. Scanlon and R.M Gunnar.
Peripartum cardiomyopathy: clinical, hemodynamic, histologic and
prognostic characteristics. J. Am. Coll. Cardiol. 8, 52-56
(1986).
110. A. Cenac, Y. Gaultier, I. Soumana, Y. Harouna and M.
Develoux. [Postpartum cardiomyopathy in the Sudanese-Sahelian
area. Clinical and epidemiologic studies of 66 cases.] Arch. Mal.
Coeur. 82, 553-558 (1989). (in French)
111. W. Lee and D.B. Cotton. Peripartum cardiomyopathy:
current concepts and clinical management. Clin. Obstet. Gynecol.
32, 54-67 (1989).
112. M. Ferriere, A. Sacrez, J.B. Bouhour, J. Cassagnes, P.
Geslin, O. Dubourg, M. Komajda and M. Degeorges. [Cardiomyopathy
in the peripartum period: current aspects. A multicenter study.
11 cases] Arch. Mal. Coeur 83, 1563-1569 (1990). (in French)
113. L.T. Iseri, P. Chung and J. Tobis. Magnesium therapy for
intractable ventricular tachyarrhythmias in normomagnesemic
patients. West. J. Med. 138, 823-828 (1983).
114. Th. Dyckner and P.O. Wester.
Magnesium-electrophysiological effects. Magnesium Bull. 8,
219-222 (1986).
115. L.T. Iseri. Magnesium and cardiac arrhythmias. Magnesium
6, 266-267 (1987).
116. L. Cohen, R. Kitzes and H. Shnaider. Multifocal atrial
techycardia responsive to parenteral magnesium. Magnesium Res. 1,
239-242 (1988).
117. H.S. Rasmussen. Justification for intravenous magnesium
therapy in acute myocardial infarction. Magnesium Res. 1, 59-73
(1988).
118. G.A. Charbon. Magnesium treatment of arrhythmia: drug or
nutritional replenishment? pitfalls for the experimental design.
In Magnesium in Health and Disease (Y. Itokawa, J. Durlach Eds.)
J. Libbey, London, 1989 (Fifth Intl Mg Sympos, Kyoto, 1988), pp.
223-228.
119. A. Sjogren, L. Edvinsson and B. Fallgren. Magnesium
deficiency in coronary artery disease and cardiac arrhythmias. J.
Intern. Med. 226, 213-222 (1989).
120. D. Antoni, M. Engel and N. Gumpel. [Magnesium therapy of
supraventricular and ventricular arrhythmias.] Magnesium Bull.
11, 125-129 (1989). (in German).
121. L.T. Iseri. Role of magnesium in cardiac
tachyarrhythmias. Am. J. Cardiol. 65, 47K-50K (1990).
122. A. Keren and D. Tzivoni. Magnesium therapy in ventricular
arrhythmias. P.A.C.E. 13, 937-945 (1990).
123. F. Perticone, D. Borelli, R. Ceravolo and P.L. Mattioli.
Antiarrhythmic short-term protective magnesium treatment in
ischemic dilated cardiomyopathy. J. Am. Coll. Nutr. 9, 492-499
(1990).
124. P.T. Sager, J. Widerhorn, R. Petersen, C. Leon, E. Ryzen,
R. Rude, S.H. Rahimtoola and A.K. Bhandari. Prospective
evaluation of parenteral magnesium sulfate in the treatment of
patients with reentrant AV supraventricular tachycardia. Am.
Heart J. 119 (2 Pt 1), 308-316 (1990).
125. R.K. Mishra. Studies on experimental magnesium deficiency
in the albino rat. 1. Functional and morphological changes
associated with low intake of magnesium. Rev. Canad. Biol. 19,
122-135 (1960).
126. H.A. Heggtveit. The cardiomyopathy of Mg-deficiency. In
Electrolytes and Cardiovascular Diseases. (E. Bajusz Ed.), S
Karger,Basel/NY, 1965, pp. 204-220.
127. H.A. Heggtveit, L. Herman and R.K. Mishra. Cardiac
necrosis and calcification in experimental magnesium deficiency.
A Light and electron microscopic study. Am. J. Pathol. 45,
757-782 (1964).
128. D. Lehr. The role of certain electrolytes and hormones in
disseminated myocardial necrosis. In Electrolytes and
Cardiovascular Diseases. E. Bajusz, Ed., S. Karger, Basel,
Switzerland, NY,NY 1965: pp248-273,
129. J. Wener, K.Pintar, M.A. Simon, R. Motola, R. Friedman,
A. Mayman, and R. Schucher. The effects of prolonged
hypomagnesemia on the cardiovascular system in young dogs. Am.
Heart J. 67, 221-231 (1964).
130. S. Bloom and A. Ahmad. Ca channel blockade, inhibition of
(Na,K)-ATPase, and myocardial necrosis associated with Mg
deficiency. FASEB J 2, A824 (1988).
131. S. Bloom. Magnesium deficiency cardiomyopathy. Am. J.
Cardiovasc. Path. 2, 7-17 (1988).
132. A.M Freedman, A.H. Atrakchi, M.M. Cassidy and W.B.
Weglicki. Magnesium deficiency-induced cardiomyopathy: protection
by vitamin E. Biochem. Biophys. Res. Commun. 170, 1102-1106
(1990).
133. W. Brigden. Uncommon myocardial disease: the non-coronary
cardiomyopathies. Lancet 2, 1179-1184 (1957).
134. J.J. Goodwin. Congestive and hypertrophic
cardiomyopathies. Lancet 1, 73I-739 (1970).
135. I. Kariv, B. Kreisler, L. Sherf, S. Feldman and T.
Rosenthal. Familial cardiomyopathy. A review of 11 families. Am.
J. Cardiol. 28, 693-707 (1971).
136. V.A. McKusick. A genetical view of cardiovascular
disease. Circulation 30, 326-357 (1964).
137. T.N. James. An etiologic concept concerning the obscure
myocardiopathies. Progr. Cardiovasc. Dis. 7, 43-64 (1964).
138. T.N. James. Pathology of small coronary arteries. Am. J.
Cardiol. 20, 679-691 (1967).
139. H.T. Blumenthal, M. Alex and A. Goldenberg. A study of
lesions of intramural coronary artery branches in diabetes
mellitus. Arch. Path. 70, 27-42 (1960).
140. S. Rubler, H. Dlugash, Y.Z. Yuceoglu, T. Kumral, A.W.
Branwood and A. Grishman. A new type of cardiomyopathy associated
with diabetic glomerulosclerosis. Am. J. Cardiol. 30, 595-602
(1972). 141. R.I. Hamby, S. Zoneraich and L. Sherman. Diabetic
cardiomyopathy. J. Am. Med. Assoc. 229, 1749-1754 (1974).
142. H.E. Martin and M. Wertman. Serum potassium, magnesium,
and calcium levels in diabetic acidosis. J. Clin. Investig.
26:217-228, 1947.
143. A.G. Beckett and J.G. Lewis. Serum magnesium in diabetes
mellitus. Clin. Sci. 18, 597-604 (1959).
144. Bachem M.G., B. Strobel, U. Jastram, E.-G. Janssen, and
Paschen K. (Magnesium and diabetes) Magnesium Bull. 2, 35-39
(1980) (in German)
145. P. Fort. Magnesium and diabetes mellitus. In Clinical
Disorders in Pediatric Nutrition. (F. Lifshitz, Ed.) Marcel
Dekker, N.Y., N.Y., 1982, pp.223-240.
146. P. Fort and F. Lifshitz. Magnesium status in children
with insulin- dependent diabetes mellitus. J. Am. Coll. Nutr. 5,
69-78 (1986).
147. Y. Rayssiguier. Lipoprotein metabolism: Importance of
magnesium. Magnesium Bull. 8, 86-193, (1986).
148. Y. Rayssiguier and E. Gueux. Magnesium and Lipids in
Cardiovascular Disease. J. Am . Coll. Nutr. 5, 507-519
(1986).
149. Y. Rayssiguier, A. Mazur, P. Cardot and E. Gueux. Effects
of magnesium on lipid metabolism and cardiovascular disease. In
Magnesium in Health and Disease (Y. Itokawa, J. Durlach Eds.) J.
Libbey, London, 1989 (Fifth Intl Mg Sympos, Kyoto, 1988), pp.
199-207.
150. R.A. Anderson. Chromium. In Modern Nutrition in Health
and Disease. M.E. Shils and V. R. Young, Eds. Lea & Febiger,
Philadelphia, PA, 1988. pp. 268-273.
151. R.A. Anderson. Recent advances in the role of chromium in
human health and diseases. In Essential and Toxic Trace Elements
in Human Health and Disease. A.S. Prasad, Ed. Publ.Alan R. Liss,
Inc. 1988, pp 189-197.
152. R.A. Anderson. Chromium Metabolism and its Role in
Disease Processes in Man. Clin. Physiol. Biochem. 4, 31-41,
(1986).
153. T. Tuvemo and M. Gebre-Medhin. The role of trace elements
in juvenile diabetes mellitus. Pediatrician 12, 213-219
(1983-85).
154. A. Jervell and F. Lange-Nielsen. Congenital deaf-mutism,
functional heart disease with prolongation of the Q-T interval,
and sudden death. Am. Heart J. 4, 59-68 (1957).
155. G.R. Fraser, P. Froggatt and T.N James. Congenital
deafness associated with electrocardiographic abnormalities. A
recessive syndrome. Quart. J. Med. 33, 361-384 (1964).
156. T.N. James. Congenital deafness and cardiac arrhythmias.
Am. J. Cardiol. 19, 627-643 (1967).
157. G.T. Koroxenidis, N.C. Webb Jr., B.B. Moschos and P.H.
Lehan. Congenital heart disease, deaf-mutism, and associated
somatic malformations in several members of one family. Am. J.
Med. 40, 149-155 (1966).
158. R.D. Spellberg. Familial sinus node disease. Chest 60,
246-251 (1971).
159. E.C. Mathews Jr., A.W. Blount Jr. and J.I. Townsend. Q-T
prolongation and ventricular arrhythmias, with and without
deafness in the same family. Am. J. Cardiol. 29, 702-711
(1972).
160. K.B. Franz. Hearing thresholds of rats fed different
levels of magnesium for two weeks. J. Am. Coll. Nutr. 11, 612
(1992).
161. H. Ising, M. Handrock, T. Guenther, R. Fischer and
Combrowski. Increased noise trauma in guinea pigs through
magnesium deficiency. Acta Otorhino- laryngol. 236, 139-146
(1982).
162. Z. Joachim, H. Ising and T. Guenther. Biochemical
mechanisms affecting susceptibility to noise-induced hearing
loss. In Noise as a Public Health Problem (G. Rossi, ed.) Proc.
of 4th Intl. Congr. vol 1, Techn. Centro Ricerde Studi Amplifer.
Milano, 1983, pp.243-255.
163. T. Guenther, H. Ising and Z. Joachims. Biochemical
mechanisms affecting susceptibility to noise-induced hearing
loss. Am. J. Otol. 10, 36-41 (1989).
164. S.H. Appel and A.D. Roses. The muscular dystrophies. In
The Metabolic Basis of Inherited Disease. (J.B. Stanbury, J.B.
Wyngaarden, D.S. Fredrickson, J.L.Goldstein and M.S. Brown Eds.)
McGraw Hill Book Co. New York, 1983, pp. 1470-1495
165. T.N. James. Observations on the cardiovascular
involvement (including the cardiac conduction system) in
progressive muscular dystrophy. Am. Heart J. 63, 48-56
(1962).
166. P.L. Wahi, S.S. Manchanda, M.S. Thind and M. Akhtar.
Cardiopathy in muscular dystrophy. Dis. Chest 53, 79-84
(1968.
167. H.L. Smith, R.L. Fischer and J.N. Etteldorf. Studies of
serum calcium and magnesium in muscular dystrophy. Am. J. Dis.
Child. 100, 714-715 (1960).
168. H.L. Smith, R.L Fischer and J.N. Etteldorf. Magnesium and
calcium in muscular dystrophy. Am. J. Dis. Child. 103, 771-776
(1962).
169. S.W. Boellner, E.J. Olson, D. Fredrickson and E.R.
Hughes. Plasma and erythrocyte magnesium in muscular dystrophy.
Am. J. Dis. Child. 110, 172- 175 (1965).
170. Bertorini, F. Cornelio, S.K. Bhattacharya, G.M. Palmieri,
I. Dones, F. Dworzak and B. Brambati. Calcium and magnesium
content in fetuses at risk and prenecrotic Duchenne muscular
dystrophy. Neurology 34, 1436-1440 (1984).
171. R.S. Shalev, O. Shalev, N. Amir, S. Porat and G.
Fawlewski-De-Leon G. Erythrocyte (Ca2+ + Mg2+)-ATPase activity
and calcium homeostasis in Duchenne muscular dystrophy. J.
Neurol. Sci. 63, 325-30 (1984)170. T.E.
172. J.F. Sundermeyer, S. Gudbjarnson, V.E. Wendt, P.B.
denBakker and R.J. Bing. Myocardial metabolism in progressive
muscular dystrophy. Circulation 24, 348-1355 (1961).
173. T.L. Wright, J.A. O'Neill and W.H. Olson. Abnormal
intrafibrillar monoamines in sex-linked muscular dystrophy.
Neurology 23, 510-517 (1973).
174. E. Bajusz, F. Homburger, J.R. Baker and L.H. Opie. The
heart muscle in muscular dystrophy with special reference to
involvement of the cardiovascular system in the hereditary
myopathy of the hamster. Ann. N. Y. Acad. Sci. 138, 213-231
(1966).
175. E. Bajusz and K. Lossnitzer. A new disease model of
chronic congestive heart failure: studies on its pathogenesis.
Trans. N.Y. Acad. Sci. Ser. II 30, 939-948 (1968).
176. B.B. Nadkarni, B. Hunt and H.A. Heggtveit. Early
ultrastructural and biochemical changes in the myopathic hamster
heart. In Myocardiology. Recent Advances in Studies on Cardiac
Metabolism. (E. Bajusz and G. Rona, Eds.) Univ. Park Press ,
Baltimore MD, 1972, pp 251-261.
177. A.J. Crawford, S.K. Bhattacharya. Excessive intracellular
zinc accumulation in cardiac and skeletal muscles of dystrophic
hamsters. Exp. Neurol. 95, 265-276 (1987).
178. J.F. Dunn and G.K. Radda. Total ion content of skeletal
and cardiac muscle in the mdx mouse dystrophy: Ca2+ is elevated
at all ages. J. Neurol. Sci. 103, 226-231 (1991).
179. M.J. Glesby, E. Rosenmann, E.G. Nylen, K. Wrogemann.
Serum CK, calcium, magnesium, and oxidative phosphorylation in
mdx mouse muscular dystrophy. Muscle Nerve 11, 852-856
(1988).
180. B.E. Jacobson, C.G. Lundquist, T.J. Griffith. Defective
respiration and oxidative phosphorylation in muscle mitochondria
of hamsters in the late stages of hereditary muscular dystrophy.
Canad, J. Biochem. 48, 1037- 1042 (1970).
181. K. Wrogemann, M.C. Blanchaer, B.E. Jacobson. A
magnesium-responsive defect of respiration and oxidative
phosphorylation in skeletal muscle mitochondria of dystrophic
hamsters. Canad. J. Biochem. 48, 1332-1338 (1970).
182. K. Wrogeman, M.C. Blanchaer, B.E. Jacobson. Abnormal
oxidative phosphorylation in skeletal muscle mitochondria of the
BIO14.6 dystrophic Syrian hamster. In Myocardiology. Recent
Advances in Studies on Cardiac Metabolism. (E. Bajusz and G.
Rona, Eds.) Univ. Park Press, Baltimore MD, 1972, pp 289-293.
183. E.T. Angelakos, L.C. Carrollo, J.B. Daniels, M.P. King
and E, Bajusz. Adrenergic neurohumors in the heart of hamsters
with hereditary myopathy during cardiac hypertrophy and failure.
In Myocardiology. Recent Advances in Studies on Cardiac
Metabolism. (E. Bajusz and G. Rona, Eds.) Univ. Park Press ,
Baltimore MD, 1972, pp 263-278.
184. W.W. Douglas and R.P. Rubin. The effects of alkaline
earths and other divalent cations on adrenal medullary secretion.
J. Physiol. 175, 231-241 (1964).
185. Bouillin D.J. The action of extracellular cations on the
release of the sympathetic transmitter from peripheral nerves. J.
Physiol. 189, 85-99 (1967).
186. J.S. Nelson. Pathology of vitamin E deficiency. In
Vitamin E. A Comprehensive Treatise. (L.J. Machlin, Ed.) Marcel
Dekker, Inc. N.Y. 1980, pp. 397-428.
187. W.O. Fenn and M. Goettsch. Electrolytes in nutritional
dystrophy in rabbits. J. Biol. Chem. 120, 41-59 (1937).
188. K.L. Blaxter and W. A. Wood. The nutrition of the young
Ayrshire calf. Composition of the tissues of normal and
dystrophic calves. Brit. J. Nutr. 6, 144-163 (1952).
189. L. Zuckerman and G.H. Marquardt. Muscle, erythrocyte and
plasma electrolytes and some other muscle constituents of rabbits
with nutritional muscular dystrophy. Proc. Soc. Exp. Biol. Med.
112, 609-610(1963).
190. L.A. Goldsmith. Relative magnesium deficiency in the rat.
J. Nutr. 98, 87-102 (1967).
191. K. Schwarz. Vitamin E, trace elements and sulfydryl
groups in respiratory decline. Vitamins and Hormones 20, 463-484
(1962).
192. A.L. Tappel. Vitamin E as the biological lipid
antioxidant. Vitamins and Hormones 20, 493-510 (1962).
193. T. Guenther and V. Hoellriegl. Increasedlipid
peroxidation in liver mitochondria from Mg-deficient rats. J.
Trace Elem. Electrol. Hlth. Dis. 3, 213-216 (1989).
194. T. Guenther, J. Vormann, V. Hoellriegl and R. Gossrau.
Effect of Mg deficiency and salicylate on lipid peroxidation in
vivo. Magnesium Bull. 13, 26-29 (1991).
195. T. Guenther, J. Vormann, V. Hoellriegl, G. Disch and H.G.
Classen. Role of lipid peroxidation and vitamin E in magnesium
deficiency. Magnesium Bull. 14, 57-66 (1992).
196. A.M Freedman, M.M. Cassidy and W.B. Weglicki. Magnesium
deficient myocardium demonstrates an increased susceptibility to
an in vivo oxidative stress. Magnesium Res. 4, 185-189
(1991).
197. H.H. Draper. Nutrient interrelationships. In Vitamin E. A
Comprehensive Treatise. (L.J. Machlin, Ed.) Marcel Dekker, Inc.
N.Y. 1980, pp. 272-288. 198. O.A. Levander. Clinical consequences
of low selenium intake and its relationship to vitamin E. Ann.
N.Y. Acad. Sci. 394, 70-82 (1982).
199. O.A. Levander. Selenium. In Modern Nutrition in Health
and Disease. M.E. Shils and V. R. Young, Eds. Lea &Febiger,
Philadelphia, PA, 1988. pp. 263-267.
200. M.J. Jackson and R.H.T Edwards. Selenium, vitamin E, free
radicals and muscle disease. Curr. Top. Nutr. Dis. 18:431-439,
1986.
201. G. Lockitch. Selenium: clinical significance and
analytical concepts. Crit. Rev. Clin. Lab. Sci. 27, 483-541
(1989).
202. M.A. Banks, W.G. Martin and D.E. Hinton. Vitamin E
deficiency in the adult Syrian golden hamster. J Nutr Growth
Cancer 4:91-108, 1987.
203. J.R. Arthur. Effects of selenium and vitamin E status on
plasma creatine kinase activity in calves. J. Nutr. 118:747-755,
1988.
204. P. Larvor. (Relations between plasma composition and
symptoms of grass tetany in bovines). Ann. Zootech. 11, 135-149
(1962). (in French)
205. Y. Rayssiguier and P. Larvor. Hypomagnesemia following
stimulation of lipolysis in ewes: effects of cold exposure and
fasting. In Magnesium in Health and Disease (M. Cantin, M.S.
Seelig, Eds.) Spectrum, N.Y, 1980. (2nd Intl. Mg Sympos. Quebec,
1976) pp. 67-72.
206. L.J. Machlin and E. Gabriel. Interactions of vitamin E
with vitamin C, vitamin B12 and zinc. In Micronutrient
Interactions: Vitamins, Minerals and Hazardous Elements. Ann.
N.Y. Acad. Sci. 355, 98-108 (1980).
207. R.C. Talamo, B.J. Rosenstein and R.W. Berninger. Cystic
fibrosis. In The Metabolic Basis of Inherited Disease. (J.B.
Stanbury, J.B. Wyngaarden, D.S. Fredrickson, J.L.Goldstein and
M.S. Brown Eds.) McGraw Hill Book Co. New York, 1983, pp.
1889-1917.
208. H. Shwachman, M. Kowalski, K.T. Khaw. Cystic fibrosis: a
new outlook. 70 patients above 25 years of age. Medicine 56,
129-149 (1977).
209. P.M. Farrell. Deficiency states, pharmacologic effects,
and nutrient requirements. In Vitamin E. A Comprehensive
Treatise. (L.J. Machlin, Ed.) Marcel Dekker, Inc. N.Y. 1980, pp.
520-620.
210. P.M. Farrell. Vitamin E. In Modern Nutrition in Health
and Disease. M.E. Shils and V. R. Young, Eds. Lea & Febiger,
Philadelphia, PA, 1988. pp. 340-354.
211. R. Sacrez, F. Klein, B. Hoffmann, J.M. Levy, J. Geisert
and R. Korn. (Hypoplasia of exocrine pancreas, associated with
endomyocardial fibrosis in one of two brothers.) Ann. Pediatrie
16, 43-48 (1969). (In French)
212. J. Kuhn, B.J. Rosestein and E.H. Oppenheimer. Metastatic
calcification in cystic fibrosis. A report of two cases.
Radiology 97, 59-64 (1970).
213. T. Foucard, M. Gebre-Medhin, K.H. Gustavson and U. Lindh.
Low concentrations of sodium and magnesium in erythrocytes from
cystic fibrosis heterozygotes. Acta Paediat. Scand. 80, 57-61
(1991).
214. L. Kopito and H. Schwachman. Spectroscopic Analysis of
Tissues from Patients with Cystic Fibrosis and Controls. Nature
202, 501-502, (1964). 215. L. Paunier, E. Girardin, P.G.
Sizonenko, M. Wyss and A. Megevand. Calcium and magnesium
concentration in sweat of normal children and patients with
cystic fibrosis. Pediatrics 52, 446-448 (1973).
216. L. Kopito, E. Elian and H. Shwachman. Sodium, potassium,
calcium, and magnesium in hair from neonates with cystic fibrosis
and in amniotic fluid from mothers of such children. Pediatrics
49, 620-624 (1972).
217. S.R. Orenstein and D.M. Orenstein. Magnesium deficiency
in cystic fibrosis. South. Med. J. 76, 1586 (1983).
218. C. Godson, M.P. Ryan, H.R. Brady, S. Bourke and M.X.
FitzGerald. Acute hypomagnesaemia complicating the treatment of
meconium ileus equivalent in cystic fibrosis. Scand. J.
Gastroenterol. Suppl143, 148-150 (1988).
219. M.A. Banks, W.G. Martin and D.E. Hinton. Long-term
histological observations in the liver and pancreas of vitamin E
and selenium deficient adult Syrian golden hamster. J Nutr Growth
Cancer 4, 109-128 (1987).
220. S.H.Mudd and H.L. Levy. Disorders of transsulfuration. In
The Metabolic Basis of Inherited Disease. (J.B. Stanbury, J.B.
Wyngaarden, D.S. Fredrickson, J.L.Goldstein and M.S. Brown Eds.)
McGraw Hill Book Co. New York, 1983, pp. 522-559.
221. L. Laster, G.L. Spaeth, S.H. Mudd and J.D. Finkelstein.
Homocystinuria due to cystathionine synthase deficiency.
(Combined Staff Conf NIH) Ann Intern Med 63:1117-1142, 1965.
222. G.W. Frimpter, R.J. Andelman and W.F. George. Vitamin B6
- dependency syndromes. New horizons in nutrition. Am. J. Clin.
Nutr. 22, 794-805 (1969).
223. J.B. Gibson, N.A.J. Carson and D.W. Neill Pathological
findings in homocystinuria. J Clin Path 17:427-437, 1964.
224. B. Wilcken, B. Turner. Homocystinuria. Reduced folate
levels during pyridoxine treatment. Arch Dis Childh 48, 58-62
(1973).
225. A. White, P. Handler, E.L. Smith, R.L. Hill and I. R.
Lehman. Principles of Biochemistry. 6th Ed. McGraw-Hill Book Co,
N.Y. 1978.
226. J.K. Aikawa. Effects of pyridoxine and desoxypyridoxine
on magnesium metabolism in the rabbit. Proc. Soc. Exp. Biol. Med.
104, 461-463 (1960).
227. D.B. McCormick, M.E. Gregory, E.E. Snell. Pyridoxal
phosphokinases. I. Assay, distribution and properties. J. Biol.
Chem. 236:2076-2084, 1961.
228. J.M. Hsu. Zinc content in tissues of pyridoxine deficient
rats. Proc. Soc. Exp. Biol. Med. 119, 177-180 (1965).
229. J. Durlach. (Studies on synergistic mechanisms between
vitamin B6 and magnesium.) J. Med. Besancon 5, 349-359 (1969).
(in French)
230. K.S. Kubena, S.E. Edgar and J.R. Veltmann. Growth and
development in rats and deficiency of magnesium and pyridoxine.
J. Am. Coll. Nutr. 7, 317-324 (1988).
231. P. Majumdar, M. Boylan, J.A. Driskell. Effect of
pyridoxine hydrochloride on tissue magnesium levels in the rat.
FASEB J. 2, A441 (1988)
232. A.E. Harriman. Dietary pyridoxine loadings affect
incidence of "spontaneous" seizures among magnesium-deprived
Mongolian gerbils (Meriones unguiculatus). Percept. Motor Skills
66, 327-337 (1988).
233. L.M. Boylan, J.E. Spallholz. Ultraviolet and fluorometric
spectral evidence for the in vitro binding of zinc and magnesium
to vitamin B-6. FASEB J. 3, 669 (1989).
This page was first uploaded to The Magnesium Web Site on
October 18, 1995
http://www.mgwater.com/



